Involvement of G6PD5 in ABA response during seed germination and root growth in Arabidopsis
- PMID: 30700259
- PMCID: PMC6354342
- DOI: 10.1186/s12870-019-1647-8
Involvement of G6PD5 in ABA response during seed germination and root growth in Arabidopsis
Abstract
Background: Glucose-6-phosphate dehydrogenase (G6PDH or G6PD) functions in supply of NADPH, which is required for plant defense responses to stresses. However, whether G6PD functions in the abscisic acid (ABA) signaling pathway remains to be elucidated. In this study, we investigated the involvement of the cytosolic G6PD5 in the ABA signaling pathway in Arabidopsis.
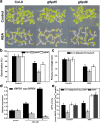
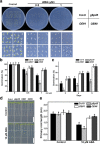
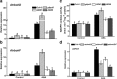
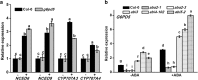
Results: We characterized the Arabidopsis single null mutant g6pd5. Phenotypic analysis showed that the mutant is more sensitive to ABA during seed germination and root growth, whereas G6PD5-overexpressing plants are less sensitive to ABA compared to wild type (WT). Furthermore, ABA induces excessive accumulation of reactive oxygen species (ROS) in mutant seeds and seedlings. G6PD5 participates in the reduction of H2O2 to H2O in the ascorbate-glutathione cycle. In addition, we found that G6PD5 suppressed the expression of Abscisic Acid Insensitive 5 (ABI5), the major ABA signaling component in dormancy control. When G6PD5 was overexpressed, the ABA signaling pathway was inactivated. Consistently, G6PD5 negatively modulates ABA-blocked primary root growth in the meristem and elongation zones. Of note, the suppression of root elongation by ABA is triggered by the cell cycle B-type cyclin CYCB1.
Conclusions: This study showed that G6PD5 is involved in the ABA-mediated seed germination and root growth by suppressing ABI5.
Keywords: Abscisic acid; Germination; Glucose-6-phosphate dehydrogenase 5; NADPH oxidases; Reactive oxygen species; Root system architecture.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Similar articles
-
Cytosolic Glucose-6-Phosphate Dehydrogenase Is Involved in Seed Germination and Root Growth Under Salinity in Arabidopsis.Front Plant Sci. 2019 Feb 22;10:182. doi: 10.3389/fpls.2019.00182. eCollection 2019. Front Plant Sci. 2019. PMID: 30873191 Free PMC article.
-
Arabidopsis VQ18 and VQ26 proteins interact with ABI5 transcription factor to negatively modulate ABA response during seed germination.Plant J. 2018 Aug;95(3):529-544. doi: 10.1111/tpj.13969. Epub 2018 Jun 8. Plant J. 2018. PMID: 29771466
-
AtPER1 enhances primary seed dormancy and reduces seed germination by suppressing the ABA catabolism and GA biosynthesis in Arabidopsis seeds.Plant J. 2020 Jan;101(2):310-323. doi: 10.1111/tpj.14542. Epub 2019 Oct 22. Plant J. 2020. PMID: 31536657
-
ABA Metabolism and Homeostasis in Seed Dormancy and Germination.Int J Mol Sci. 2021 May 11;22(10):5069. doi: 10.3390/ijms22105069. Int J Mol Sci. 2021. PMID: 34064729 Free PMC article. Review.
-
Progress of ABA function in endosperm cellularization and storage product accumulation.Plant Cell Rep. 2024 Nov 20;43(12):287. doi: 10.1007/s00299-024-03378-6. Plant Cell Rep. 2024. PMID: 39565413 Review.
Cited by
-
ABA Enhances Drought Resistance During Rapeseed (Brassica napus L.) Seed Germination Through the Gene Regulatory Network Mediated by ABA Insensitive 5.Plants (Basel). 2025 Apr 22;14(9):1276. doi: 10.3390/plants14091276. Plants (Basel). 2025. PMID: 40364305 Free PMC article.
-
Roles of Reactive Oxygen Species and Mitochondria in Seed Germination.Front Plant Sci. 2021 Dec 9;12:781734. doi: 10.3389/fpls.2021.781734. eCollection 2021. Front Plant Sci. 2021. PMID: 34956279 Free PMC article. Review.
-
Updated role of ABA in seed maturation, dormancy, and germination.J Adv Res. 2021 Mar 31;35:199-214. doi: 10.1016/j.jare.2021.03.011. eCollection 2022 Jan. J Adv Res. 2021. PMID: 35003801 Free PMC article. Review.
-
Gm6PGDH1, a Cytosolic 6-Phosphogluconate Dehydrogenase, Enhanced Tolerance to Phosphate Starvation by Improving Root System Development and Modifying the Antioxidant System in Soybean.Front Plant Sci. 2021 Aug 13;12:704983. doi: 10.3389/fpls.2021.704983. eCollection 2021. Front Plant Sci. 2021. PMID: 34484268 Free PMC article.
-
Genome-Wide Investigation of G6PDH Gene in Strawberry: Evolution and Expression Analysis during Development and Stress.Int J Mol Sci. 2022 Apr 25;23(9):4728. doi: 10.3390/ijms23094728. Int J Mol Sci. 2022. PMID: 35563120 Free PMC article.
References
-
- Esposito S, Massaro G, Vona V, Di Martino Rigano V, Carfagna S. Glutamate synthesis in barley roots: the role of the plastidic glucose-6-phosphate dehydrogenase. Planta. 2003;216(4):639–647. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

