The spleen may be an important target of stem cell therapy for stroke
- PMID: 30700305
- PMCID: PMC6352449
- DOI: 10.1186/s12974-019-1400-0
The spleen may be an important target of stem cell therapy for stroke
Abstract
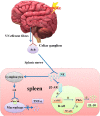
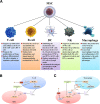
Stroke is the most common cerebrovascular disease, the second leading cause of death behind heart disease and is a major cause of long-term disability worldwide. Currently, systemic immunomodulatory therapy based on intravenous cells is attracting attention. The immune response to acute stroke is a major factor in cerebral ischaemia (CI) pathobiology and outcomes. Over the past decade, the significant contribution of the spleen to ischaemic stroke has gained considerable attention in stroke research. The changes in the spleen after stroke are mainly reflected in morphology, immune cells and cytokines, and these changes are closely related to the stroke outcomes. Autonomic nervous system (ANS) activation, release of central nervous system (CNS) antigens and chemokine/chemokine receptor interactions have been documented to be essential for efficient brain-spleen cross-talk after stroke. In various experimental models, human umbilical cord blood cells (hUCBs), haematopoietic stem cells (HSCs), bone marrow stem cells (BMSCs), human amnion epithelial cells (hAECs), neural stem cells (NSCs) and multipotent adult progenitor cells (MAPCs) have been shown to reduce the neurological damage caused by stroke. The different effects of these cell types on the interleukin (IL)-10, interferon (IFN), and cholinergic anti-inflammatory pathways in the spleen after stroke may promote the development of new cell therapy targets and strategies. The spleen will become a potential target of various stem cell therapies for stroke represented by MAPC treatment.
Keywords: IL-10; Multipotent adult progenitor cells; Spleen; Stem cells; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Similar articles
-
Multipotent Adult Progenitor Cells Enhance Recovery After Stroke by Modulating the Immune Response from the Spleen.Stem Cells. 2017 May;35(5):1290-1302. doi: 10.1002/stem.2600. Epub 2017 Mar 6. Stem Cells. 2017. PMID: 28263009
-
Transplantation of bone marrow-derived stem cells: a promising therapy for stroke.Cell Transplant. 2007;16(2):159-69. Cell Transplant. 2007. PMID: 17474297 Review.
-
Recent Advances in Mono- and Combined Stem Cell Therapies of Stroke in Animal Models and Humans.Int J Mol Sci. 2019 Nov 29;20(23):6029. doi: 10.3390/ijms20236029. Int J Mol Sci. 2019. PMID: 31795466 Free PMC article. Review.
-
Interferon-γ Promotes Neuronal Repair by Transplanted Neural Stem Cells in Ischemic Rats.Stem Cells Dev. 2018 Mar 1;27(5):355-366. doi: 10.1089/scd.2017.0225. Epub 2018 Feb 15. Stem Cells Dev. 2018. PMID: 29298609
-
Biotherapies in stroke.Rev Neurol (Paris). 2014 Dec;170(12):779-98. doi: 10.1016/j.neurol.2014.10.005. Epub 2014 Nov 6. Rev Neurol (Paris). 2014. PMID: 25459115 Review.
Cited by
-
Human umbilical cord-derived mesenchymal stem cells alleviate insulin resistance in diet-induced obese mice via an interaction with splenocytes.Stem Cell Res Ther. 2022 Mar 21;13(1):109. doi: 10.1186/s13287-022-02791-6. Stem Cell Res Ther. 2022. PMID: 35313972 Free PMC article.
-
Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut-Immune-Brain Axis.Int J Mol Sci. 2022 Sep 23;23(19):11224. doi: 10.3390/ijms231911224. Int J Mol Sci. 2022. PMID: 36232524 Free PMC article.
-
Spleen participation in partial MHC class II construct neuroprotection in stroke.CNS Neurosci Ther. 2020 Jul;26(7):663-669. doi: 10.1111/cns.13369. Epub 2020 Mar 31. CNS Neurosci Ther. 2020. PMID: 32237074 Free PMC article. Review.
-
Umbilical mesenchymal stem cells mitigate T-cell compartments shift and Th17/Treg imbalance in acute ischemic stroke via mitochondrial transfer.Stem Cell Res Ther. 2025 Mar 12;16(1):134. doi: 10.1186/s13287-025-04224-6. Stem Cell Res Ther. 2025. PMID: 40075467 Free PMC article.
-
Potential of stem cell therapy in intracerebral hemorrhage.Mol Biol Rep. 2020 Jun;47(6):4671-4680. doi: 10.1007/s11033-020-05457-9. Epub 2020 May 15. Mol Biol Rep. 2020. PMID: 32415506 Review.
References
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- Maya-Espinosa G, Collazo-Navarrete O, Millán-Aldaco D, Palomero-Rivero M, Guerrero-Flores G, Drucker-Colín R, et al. Mouse embryonic stem cell-derived cells reveal niches that support neuronal differentiation in the adult rat brain. Stem Cells. 2015;33(2):491–502. doi: 10.1002/stem.1856. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

