Efficacy and cost-effectiveness of Internet-based selective eating disorder prevention: study protocol for a randomized controlled trial within the ProHEAD Consortium
- PMID: 30700318
- PMCID: PMC6354385
- DOI: 10.1186/s13063-018-3161-y
Efficacy and cost-effectiveness of Internet-based selective eating disorder prevention: study protocol for a randomized controlled trial within the ProHEAD Consortium
Abstract
Background: The development of efficacious, cost-effective, and widely accessible programs for the prevention of eating disorders (EDs) is crucial in order to reduce the ED-related burden of illness. Programs using dissonance-based and cognitive behavioral approaches are most effective for the selective prevention of ED. Internet-based delivery is assumed to maximize the reach and impact of preventive efforts. However, the current evidence for Internet-based ED prevention is limited. The present trial evaluates the efficacy and cost-effectiveness of two new interventions (based on dissonance theory and principles of cognitive behavioral therapy (CBT)) that are implemented as add-ons to the existing Internet-based ED prevention program ProYouth.
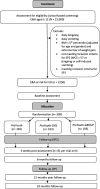
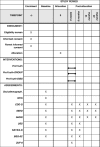
Methods: The trial is one of five sub-projects of the German multicenter consortium ProHEAD. It is a three-arm, parallel, randomized controlled superiority trial. Participants will be randomized to (1) the online program ProYouth (active control condition) or (2) ProYouth plus a structured dissonance-based module or (3) ProYouth plus a CBT-based chat group intervention. As part of ProHEAD, a representative school-based sample of N = 15,000 students (≥ 12 years) will be screened for mental health problems. N = 309 participants at risk for ED (assessed with the Weight Concerns Scale (WCS) and the Short Evaluation of Eating Disorders (SEED)) will be included in the present trial. Online assessments will be conducted at baseline, at end of intervention (6 weeks), at 6 months follow-up, and - as part of ProHEAD - at 12 and 24 months follow-up. The primary outcome is ED-related impairment (assessed with the Child version of the Eating Disorder Examination-Questionnaire (ChEDE-Q)) at the end of the intervention. Secondary outcomes include ED-related symptomatology at follow-up, ED-related stigma, ED-related help-seeking, and acceptance of and compliance with the interventions. For the health economic evaluation data on costs of the interventions, healthcare utilization and health-related quality of life will be assessed.
Discussion: This is the first study augmenting a flexible prevention approach such as ProYouth with structured evidence-based modules in order to overcome some of the key limitations in the current practice of ED prevention.
Trial registration: German Clinical Trials Register (DRKS), DRKS00014679 . Registered on 25 April 2018.
Keywords: CBT; Dissonance; Eating disorders; Internet; Prevention; ProHEAD.
Conflict of interest statement
Ethics approval and consent to participate
The ProHEAD Consortium including the present trial was reviewed and approved by the ethics committee of the University Hospital Heidelberg, on April, 19 (reference number: S-086/2018). Subsequently, ethical approval is sought at all other study sites recruiting participants (Hamburg, Leipzig, Marburg, Schwäbisch Gmünd).
The trial will follow the Declaration of Helsinki and the Guidelines for Good Clinical Practice (ICH-GCP) in their latest revision. Written informed consent and assent will be obtained from all participating C&A and from at least one of their primary caregivers. The Internet-based platforms to assess data and to deliver the interventions will follow latest regulations concerning data security. All collected information will be confidential and held in accordance with ICH-GCP. Participants will be free to withdraw consent from the study at any time without stating reasons.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
School-based mental health promotion in children and adolescents with StresSOS using online or face-to-face interventions: study protocol for a randomized controlled trial within the ProHEAD Consortium.Trials. 2019 Jan 18;20(1):64. doi: 10.1186/s13063-018-3159-5. Trials. 2019. PMID: 30658675 Free PMC article.
-
Efficacy and cost-effectiveness of two online interventions for children and adolescents at risk for depression (E.motion trial): study protocol for a randomized controlled trial within the ProHEAD consortium.Trials. 2019 Jan 15;20(1):53. doi: 10.1186/s13063-018-3156-8. Trials. 2019. PMID: 30646944 Free PMC article.
-
An Internet-based intervention for eating disorders consisting of automated computer-tailored feedback with or without supplemented frequent or infrequent support from a coach: study protocol for a randomized controlled trial.Trials. 2013 Oct 17;14:340. doi: 10.1186/1745-6215-14-340. Trials. 2013. PMID: 24135131 Free PMC article. Clinical Trial.
-
Internet eating disorder prevention.Curr Opin Psychiatry. 2018 Nov;31(6):456-461. doi: 10.1097/YCO.0000000000000450. Curr Opin Psychiatry. 2018. PMID: 30234524 Review.
-
[Prevention of eating disorder: a review].Z Kinder Jugendpsychiatr Psychother. 2017 Sep;45(5):403-413. doi: 10.1024/1422-4917/a000506. Epub 2016 Dec 13. Z Kinder Jugendpsychiatr Psychother. 2017. PMID: 27951744 Review. German.
Cited by
-
Reasons for non-participation of children and adolescents in a large-scale school-based mental health project.Front Public Health. 2024 Jan 8;11:1294862. doi: 10.3389/fpubh.2023.1294862. eCollection 2023. Front Public Health. 2024. PMID: 38259782 Free PMC article.
-
School-based mental health promotion in children and adolescents with StresSOS using online or face-to-face interventions: study protocol for a randomized controlled trial within the ProHEAD Consortium.Trials. 2019 Jan 18;20(1):64. doi: 10.1186/s13063-018-3159-5. Trials. 2019. PMID: 30658675 Free PMC article.
-
Moderators of pre-post changes in school-based mental health promotion: Psychological stress symptom decrease for adolescents with mental health problems, knowledge increase for all.Front Psychiatry. 2022 Aug 4;13:899185. doi: 10.3389/fpsyt.2022.899185. eCollection 2022. Front Psychiatry. 2022. PMID: 35990085 Free PMC article.
-
Promoting Help-seeking using E-technology for ADolescents with mental health problems: study protocol for a randomized controlled trial within the ProHEAD Consortium.Trials. 2019 Jan 31;20(1):94. doi: 10.1186/s13063-018-3157-7. Trials. 2019. PMID: 30704534 Free PMC article.
-
Development of decision rules for an adaptive aftercare intervention based on individual symptom courses for agoraphobia patients.Sci Rep. 2024 Feb 6;14(1):3056. doi: 10.1038/s41598-024-52803-z. Sci Rep. 2024. PMID: 38321070 Free PMC article.
References
-
- Crow S. The economics of eating disorder treatment. Curr Psychiatry Rep. 2014;454. 10.1007/s11920-014-0454-z. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

