Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via PEAR1 and GPIbα
- PMID: 30700416
- PMCID: PMC6373755
- DOI: 10.1182/bloodadvances.2018024950
Synthetic glycopolymers and natural fucoidans cause human platelet aggregation via PEAR1 and GPIbα
Abstract
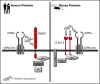
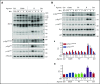
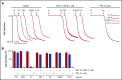
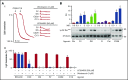
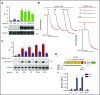
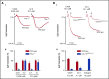
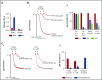
Fucoidans are sulfated fucose-based polysaccharides that activate platelets and have pro- and anticoagulant effects; thus, they may have therapeutic value. In the present study, we show that 2 synthetic sulfated α-l-fucoside-pendant glycopolymers (with average monomeric units of 13 and 329) and natural fucoidans activate human platelets through a Src- and phosphatidylinositol 3-kinase (PI3K)-dependent and Syk-independent signaling cascade downstream of the platelet endothelial aggregation receptor 1 (PEAR1). Synthetic glycopolymers and natural fucoidan stimulate marked phosphorylation of PEAR1 and Akt, but not Syk. Platelet aggregation and Akt phosphorylation induced by natural fucoidan and synthetic glycopolymers are blocked by a monoclonal antibody to PEAR1. Direct binding of sulfated glycopolymers to epidermal like growth factor (EGF)-like repeat 13 of PEAR1 was shown by avidity-based extracellular protein interaction screen technology. In contrast, synthetic glycopolymers and natural fucoidans activate mouse platelets through a Src- and Syk-dependent pathway regulated by C-type lectin-like receptor 2 (CLEC-2) with only a minor role for PEAR1. Mouse platelets lacking the extracellular domain of GPIbα and human platelets treated with GPIbα-blocking antibodies display a reduced aggregation response to synthetic glycopolymers. We found that synthetic sulfated glycopolymers bind directly to GPIbα, substantiating that GPIbα facilitates the interaction of synthetic glycopolymers with CLEC-2 or PEAR1. Our results establish PEAR1 as the major signaling receptor for natural fucose-based polysaccharides and synthetic glycopolymers in human, but not in mouse, platelets. Sulfated α-l-fucoside-pendant glycopolymers are unique tools for further investigation of the physiological role of PEAR1 in platelets and beyond.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Zhang Z, Till S, Knappe S, et al. . Screening of complex fucoidans from four brown algae species as procoagulant agents. Carbohydr Polym. 2015;115:677-685. - PubMed
-
- Prasad S, Lillicrap D, Labelle A, et al. . Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood. 2008;111(2):672-679. - PubMed
-
- Min S-K, Kwon O-C, Lee S, Park K-H, Kim J-K. An antithrombotic fucoidan, unlike heparin, does not prolong bleeding time in a murine arterial thrombosis model: a comparative study of Undaria pinnatifida sporophylls and Fucus vesiculosus. Phytother Res. 2012;26(5):752-757. - PubMed
-
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, et al. ; Consorzio Interuniversitario Nazionale per la Bio-Oncologia, Italy. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17(5):541-552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

