Performance of a Novel Low-Cost, Instrument-Free Plasma Separation Device for HIV Viral Load Quantification and Determination of Treatment Failure in People Living with HIV in Malaysia: a Diagnostic Accuracy Study
- PMID: 30700508
- PMCID: PMC6440787
- DOI: 10.1128/JCM.01683-18
Performance of a Novel Low-Cost, Instrument-Free Plasma Separation Device for HIV Viral Load Quantification and Determination of Treatment Failure in People Living with HIV in Malaysia: a Diagnostic Accuracy Study
Abstract
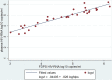
HIV viral load (VL) testing is the recommended method for monitoring the response of people living with HIV and receiving antiretroviral therapy (ART). The availability of standard plasma VL testing in low- and middle-income countries (LMICs), and access to this testing, are limited by the need to use fresh plasma. Good specimen collection methods for HIV VL testing that are applicable to resource-constrained settings are needed. We assessed the diagnostic performance of the filtered dried plasma spot (FDPS), created using the newly developed, instrument-free VLPlasma device, in identifying treatment failure at a VL threshold of 1,000 copies/ml in fresh plasma. Performance was compared with that of the conventional dried blood spot (DBS). Venous blood samples from 201 people living with HIV and attending an infectious disease clinic in Malaysia were collected, and HIV VL was quantified using fresh plasma (the reference standard), FDPS, and DBS specimens. VL testing was done using the Roche Cobas AmpliPrep/Cobas TaqMan v2.0 assay. At a threshold of 1,000 copies/ml, the diagnostic performance of the FDPS was superior (sensitivity, 100% [95% confidence interval {CI}, 89.1 to 100%]; specificity, 100% [95% CI, 97.8 to 100%]) to that of the DBS (sensitivity, 100% [95% CI, 89.4 to 100%]; specificity, 36.8% [95% CI, 29.4 to 44.7%]) (P < 0.001). A stronger correlation was observed between the FDPS VL and the plasma VL (r = 0.94; P < 0.001) than between the DBS VL and the plasma VL (r = 0.85; P < 0.001). The mean difference in VL measures between the FDPS and plasma (plasma VL minus FDPS VL) was 0.127 log10 copies/ml (standard deviation [SD], 0.32), in contrast to -0.95 log10 copies/ml (SD, 0.84) between the DBS and plasma. HIV VL measurement using the FDPS outperformed that with the DBS in identifying treatment failure at a threshold of 1,000 copies/ml and compared well with the quantification of VL in plasma. The FDPS can be an attractive alternative to fresh plasma for improving access to HIV VL monitoring among people living with HIV on ART in LMICs.
Keywords: HIV; Malaysia; diagnostic accuracy; dried blood spot (DBS); plasma separation; viral load.
Copyright © 2019 Pham et al.
Figures





Similar articles
-
HIV-1 viral load measurement in venous blood and fingerprick blood using Abbott RealTime HIV-1 DBS assay.J Clin Virol. 2017 Jul;92:56-61. doi: 10.1016/j.jcv.2017.05.002. Epub 2017 May 13. J Clin Virol. 2017. PMID: 28531553
-
Comparison of dried blood spot (DBS) and plasma HIV-1 viral load measurements using Roche COBAS AmpliPrep/COBAS TaqMan assay, Northwest Ethiopia.Virol J. 2025 May 15;22(1):145. doi: 10.1186/s12985-025-02762-2. Virol J. 2025. PMID: 40375258 Free PMC article.
-
Limited utility of dried-blood- and plasma spot-based screening for antiretroviral treatment failure with Cobas Ampliprep/TaqMan HIV-1 version 2.0.J Clin Microbiol. 2014 Nov;52(11):3878-83. doi: 10.1128/JCM.02063-14. Epub 2014 Aug 20. J Clin Microbiol. 2014. PMID: 25143579 Free PMC article.
-
The performance of using dried blood spot specimens for HIV-1 viral load testing: A systematic review and meta-analysis.PLoS Med. 2022 Aug 22;19(8):e1004076. doi: 10.1371/journal.pmed.1004076. eCollection 2022 Aug. PLoS Med. 2022. PMID: 35994520 Free PMC article.
-
HIV-1 viral load testing in resource-limited settings: Challenges and solutions for specimen integrity.Rev Med Virol. 2021 Mar;31(2):e2165. doi: 10.1002/rmv.2165. Epub 2020 Sep 25. Rev Med Virol. 2021. PMID: 32978882 Review.
Cited by
-
Clinical evaluation of patterned dried plasma spot cards to support quantification of HIV viral load and reflexive genotyping.Proc Natl Acad Sci U S A. 2025 Feb 18;122(7):e2419160122. doi: 10.1073/pnas.2419160122. Epub 2025 Feb 10. Proc Natl Acad Sci U S A. 2025. PMID: 39928862 Free PMC article.
-
Point-of-Care Diagnostics for Diagnosis of Active Syphilis Infection: Needs, Challenges and the Way Forward.Int J Environ Res Public Health. 2022 Jul 4;19(13):8172. doi: 10.3390/ijerph19138172. Int J Environ Res Public Health. 2022. PMID: 35805831 Free PMC article.
-
Switch to second-line versus continued first-line antiretroviral therapy for patients with low-level HIV-1 viremia: An open-label randomized controlled trial in Lesotho.PLoS Med. 2020 Sep 16;17(9):e1003325. doi: 10.1371/journal.pmed.1003325. eCollection 2020 Sep. PLoS Med. 2020. PMID: 32936795 Free PMC article. Clinical Trial.
-
Dried Blood Spots and Plasma Separation Cards can Broaden Access to Molecular Testing for HBV, HCV and HIV.Rev Med Virol. 2025 Sep;35(5):e70059. doi: 10.1002/rmv.70059. Rev Med Virol. 2025. PMID: 40760468 Free PMC article. Review.
-
Left behind on the path to 90-90-90: understanding and responding to HIV among displaced people.J Int AIDS Soc. 2022 Nov;25(11):e26031. doi: 10.1002/jia2.26031. J Int AIDS Soc. 2022. PMID: 36352546 Free PMC article.
References
-
- WHO. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization. Geneva, Switzerland. www.who.int/hiv/pub/guidelines/arv2013/en. - PubMed
-
- Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Jouquet G, Kerschberger B, Mekeidje C, Cyr J, Mafikudze A, Han W, Lujan J, Teck R, Antierens A, van Griensven J, Reid T. 2014. Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 67:45–51. doi: 10.1097/QAI.0000000000000224. - DOI - PMC - PubMed
-
- Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, Botes ME, Mandaliya K, Wellington M, Osibogun A, Stevens WS, van Vugt M, de Wit TR; PharmAccess African Studies to Evaluate Resistance. 2011. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr 58:23–31. doi: 10.1097/QAI.0b013e318227fc34. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

