The Gut Microbiota Is Associated with Clearance of Clostridium difficile Infection Independent of Adaptive Immunity
- PMID: 30700514
- PMCID: PMC6354811
- DOI: 10.1128/mSphereDirect.00698-18
The Gut Microbiota Is Associated with Clearance of Clostridium difficile Infection Independent of Adaptive Immunity
Abstract
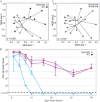
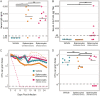
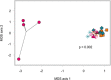
Clostridium (Clostridioides) difficile, a Gram-positive, anaerobic bacterium, is the leading single cause of nosocomial infections in the United States. A major risk factor for Clostridium difficile infection (CDI) is prior exposure to antibiotics, as they increase susceptibility to CDI by altering the membership of the microbial community enabling colonization. The importance of the gut microbiota in providing protection from CDI is underscored by the reported 80 to 90% success rate of fecal microbial transplants in treating recurrent infections. Adaptive immunity, specifically humoral immunity, is also sufficient to protect from both acute and recurrent CDI. However, the role of the adaptive immune system in mediating clearance of C. difficile has yet to be resolved. Using murine models of CDI, we found that adaptive immunity is dispensable for clearance of C. difficile However, random forest analysis using only two members of the resident bacterial community correctly identified animals that would go on to clear the infection with 66.7% accuracy. These findings indicate that the indigenous gut microbiota independent of adaptive immunity facilitates clearance of C. difficile from the murine gastrointestinal tract.IMPORTANCEClostridium difficile infection is a major cause of morbidity and mortality in hospitalized patients in the United States. Currently, the role of the adaptive immune response in modulating levels of C. difficile colonization is unresolved. This work suggests that the indigenous gut microbiota is a main factor that promotes clearance of C. difficile from the GI tract. Our results show that clearance of C. difficile can occur without contributions from the adaptive immune response. This study also has implications for the design of preclinical studies testing the efficacy of vaccines on clearance of bacterial pathogens, as inherent differences in the baseline community structure of animals may bias findings.
Keywords: Clostridium difficile; adaptive immunity; colonization resistance; intestinal colonization; microbiota.
Copyright © 2019 Leslie et al.
Figures


References
-
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
