Purine Homeostasis Is Necessary for Developmental Timing, Germline Maintenance and Muscle Integrity in Caenorhabditis elegans
- PMID: 30700528
- PMCID: PMC6456310
- DOI: 10.1534/genetics.118.301062
Purine Homeostasis Is Necessary for Developmental Timing, Germline Maintenance and Muscle Integrity in Caenorhabditis elegans
Abstract
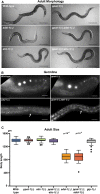
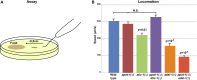
Purine homeostasis is ensured through a metabolic network widely conserved from prokaryotes to humans. Purines can either be synthesized de novo, reused, or produced by interconversion of extant metabolites using the so-called recycling pathway. Although thoroughly characterized in microorganisms, such as yeast or bacteria, little is known about regulation of the purine biosynthesis network in metazoans. In humans, several diseases are linked to purine metabolism through as yet poorly understood etiologies. Particularly, the deficiency in adenylosuccinate lyase (ADSL)-an enzyme involved both in the purine de novo and recycling pathways-causes severe muscular and neuronal symptoms. In order to address the mechanisms underlying this deficiency, we established Caenorhabditis elegans as a metazoan model organism to study purine metabolism, while focusing on ADSL. We show that the purine biosynthesis network is functionally conserved in C. elegans Moreover, adsl-1 (the gene encoding ADSL in C. elegans) is required for developmental timing, germline stem cell maintenance and muscle integrity. Importantly, these traits are not affected when solely the de novo pathway is abolished, and we present evidence that germline maintenance is linked specifically to ADSL activity in the recycling pathway. Hence, our results allow developmental and tissue specific phenotypes to be ascribed to separable steps of the purine metabolic network in an animal model.
Keywords: ADSL deficiency; AICAR; SAICAR; SZMP; ZMP; metabolism; purine salvage pathway.
Copyright © 2019 by the Genetics Society of America.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

