HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation
- PMID: 30700543
- PMCID: PMC6839735
- DOI: 10.1136/gutjnl-2018-316091
HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation
Abstract
Objective: Developing a small animal model that accurately delineates the natural history of hepatitis B virus (HBV) infection and immunopathophysiology is necessary to clarify the mechanisms of host-virus interactions and to identify intervention strategies for HBV-related liver diseases. This study aimed to develop an HBV-induced chronic hepatitis and cirrhosis mouse model through transplantation of human bone marrow mesenchymal stem cells (hBMSCs).
Design: Transplantation of hBMSCs into Fah-/-Rag2-/-IL-2Rγc-/- SCID (FRGS) mice with fulminant hepatic failure (FHF) induced by hamster-anti-mouse CD95 antibody JO2 generated a liver and immune cell dual-humanised (hBMSC-FRGS) mouse. The generated hBMSC-FRGS mice were subjected to assessments of sustained viremia, specific immune and inflammatory responses and liver pathophysiological injury to characterise the progression of chronic hepatitis and cirrhosis after HBV infection.
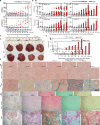
Results: The implantation of hBMSCs rescued FHF mice, as demonstrated by robust proliferation and transdifferentiation of functional human hepatocytes and multiple immune cell lineages, including B cells, T cells, natural killer cells, dendritic cells and macrophages. After HBV infection, the hBMSC-FRGS mice developed sustained viremia and specific immune and inflammatory responses and showed progression to chronic hepatitis and liver cirrhosis at a frequency of 55% after 54 weeks.
Conclusion: This new humanised mouse model recapitulates the liver cirrhosis induced by human HBV infection, thus providing research opportunities for understanding viral immune pathophysiology and testing antiviral therapies in vivo.
Keywords: chronic hepatitis; hepatitis B; liver cirrhosis; stem cells.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





Similar articles
-
Transcriptomics confirm the establishment of a liver-immune dual-humanized mouse model after transplantation of a single type of human bone marrow mesenchymal stem cell.Liver Int. 2023 Jun;43(6):1345-1356. doi: 10.1111/liv.15546. Epub 2023 Mar 8. Liver Int. 2023. PMID: 36810858
-
A Chimeric Humanized Mouse Model by Engrafting the Human Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cell for the Chronic Hepatitis B Virus Infection.Front Microbiol. 2018 May 8;9:908. doi: 10.3389/fmicb.2018.00908. eCollection 2018. Front Microbiol. 2018. PMID: 29867819 Free PMC article.
-
Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages.PLoS Pathog. 2014 Mar 20;10(3):e1004032. doi: 10.1371/journal.ppat.1004032. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651854 Free PMC article.
-
Natural history of chronic hepatitis B and C.J Gastroenterol Hepatol. 1999 May;14 Suppl:S1-5. doi: 10.1046/j.1440-1746.1999.01903.x. J Gastroenterol Hepatol. 1999. PMID: 10382630 Review.
-
Humanized chimeric mouse models of hepatitis B virus infection.Int J Infect Dis. 2017 Jun;59:131-136. doi: 10.1016/j.ijid.2017.04.002. Epub 2017 Apr 10. Int J Infect Dis. 2017. PMID: 28408253 Review.
Cited by
-
MiRNA/mRNA network topology in hepatitis virus B-related liver cirrhosis reveals miR-20a-5p/340-5p as hubs initiating fibrosis.BMC Med Genomics. 2022 Nov 14;15(1):240. doi: 10.1186/s12920-022-01390-x. BMC Med Genomics. 2022. PMID: 36376845 Free PMC article.
-
Obstacles and opportunities in the prevention and treatment of HBV-related hepatocellular carcinoma.Genes Dis. 2020 Jan 10;7(3):291-298. doi: 10.1016/j.gendis.2019.12.014. eCollection 2020 Sep. Genes Dis. 2020. PMID: 32884983 Free PMC article.
-
Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer.Cell Mol Immunol. 2021 Jan;18(1):57-72. doi: 10.1038/s41423-020-00561-z. Epub 2020 Oct 12. Cell Mol Immunol. 2021. PMID: 33041339 Free PMC article. Review.
-
Molecular Mechanisms and Animal Models of HBV-Related Hepatocellular Carcinoma: With Emphasis on Metastatic Tumor Antigen 1.Int J Mol Sci. 2021 Aug 29;22(17):9380. doi: 10.3390/ijms22179380. Int J Mol Sci. 2021. PMID: 34502289 Free PMC article. Review.
-
Potential Networks Regulated by MSCs in Acute-On-Chronic Liver Failure: Exosomal miRNAs and Intracellular Target Genes.Front Genet. 2021 Apr 23;12:650536. doi: 10.3389/fgene.2021.650536. eCollection 2021. Front Genet. 2021. PMID: 33968135 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
