Intrinsically disordered proteins in synaptic vesicle trafficking and release
- PMID: 30700558
- PMCID: PMC6416451
- DOI: 10.1074/jbc.REV118.006493
Intrinsically disordered proteins in synaptic vesicle trafficking and release
Abstract
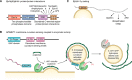
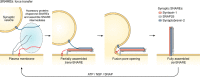
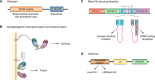
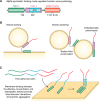
The past few years have resulted in an increased awareness and recognition of the prevalence and roles of intrinsically disordered proteins and protein regions (IDPs and IDRs, respectively) in synaptic vesicle trafficking and exocytosis and in overall synaptic organization. IDPs and IDRs constitute a class of proteins and protein regions that lack stable tertiary structure, but nevertheless retain biological function. Their significance in processes such as cell signaling is now well accepted, but their pervasiveness and importance in other areas of biology are not as widely appreciated. Here, we review the prevalence and functional roles of IDPs and IDRs associated with the release and recycling of synaptic vesicles at nerve terminals, as well as with the architecture of these terminals. We hope to promote awareness, especially among neuroscientists, of the importance of this class of proteins in these critical pathways and structures. The examples discussed illustrate some of the ways in which the structural flexibility conferred by intrinsic protein disorder can be functionally advantageous in the context of cellular trafficking and synaptic function.
Keywords: complexin; intrinsically disordered protein; lipid vesicle; membrane trafficking; membraneless organelles; neurotransmitter release; phase transitions; protein folding; synaptic vesicles; α-synuclein.
© 2019 Snead and Eliezer.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Complexin Mutants Reveal Partial Segregation between Recycling Pathways That Drive Evoked and Spontaneous Neurotransmission.J Neurosci. 2017 Jan 11;37(2):383-396. doi: 10.1523/JNEUROSCI.1854-16.2016. J Neurosci. 2017. PMID: 28077717 Free PMC article.
-
Functional unfoldomics: Roles of intrinsic disorder in protein (multi)functionality.Adv Protein Chem Struct Biol. 2024;138:179-210. doi: 10.1016/bs.apcsb.2023.11.001. Epub 2023 Nov 22. Adv Protein Chem Struct Biol. 2024. PMID: 38220424 Review.
-
Features of molecular recognition of intrinsically disordered proteins via coupled folding and binding.Protein Sci. 2019 Nov;28(11):1952-1965. doi: 10.1002/pro.3718. Epub 2019 Sep 4. Protein Sci. 2019. PMID: 31441158 Free PMC article. Review.
-
Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes.J Neurochem. 1999 Dec;73(6):2227-39. doi: 10.1046/j.1471-4159.1999.0732227.x. J Neurochem. 1999. PMID: 10582580 Review.
-
Huntingtin-associated protein-1 is a synapsin I-binding protein regulating synaptic vesicle exocytosis and synapsin I trafficking.J Neurochem. 2016 Sep;138(5):710-21. doi: 10.1111/jnc.13703. Epub 2016 Jul 18. J Neurochem. 2016. PMID: 27315547
Cited by
-
Insights into Membrane Curvature Sensing and Membrane Remodeling by Intrinsically Disordered Proteins and Protein Regions.J Membr Biol. 2022 Jun;255(2-3):237-259. doi: 10.1007/s00232-022-00237-x. Epub 2022 Apr 22. J Membr Biol. 2022. PMID: 35451616 Free PMC article. Review.
-
Inherited and Sporadic Amyotrophic Lateral Sclerosis and Fronto-Temporal Lobar Degenerations arising from Pathological Condensates of Phase Separating Proteins.Hum Mol Genet. 2019 Nov 21;28(R2):R187-R196. doi: 10.1093/hmg/ddz162. Hum Mol Genet. 2019. PMID: 31595953 Free PMC article. Review.
-
Do Changes in Synaptic Autophagy Underlie the Cognitive Impairments in Huntington's Disease?J Huntingtons Dis. 2021;10(2):227-238. doi: 10.3233/JHD-200466. J Huntingtons Dis. 2021. PMID: 33780373 Free PMC article. Review.
-
Physiological and pathological effects of phase separation in the central nervous system.J Mol Med (Berl). 2024 May;102(5):599-615. doi: 10.1007/s00109-024-02435-7. Epub 2024 Mar 5. J Mol Med (Berl). 2024. PMID: 38441598 Free PMC article. Review.
-
Synaptic sabotage: How Tau and α-Synuclein undermine synaptic health.J Cell Biol. 2025 Feb 3;224(2):e202409104. doi: 10.1083/jcb.202409104. Epub 2024 Dec 24. J Cell Biol. 2025. PMID: 39718548 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

