Zinc binding regulates amyloid-like aggregation of GAPR-1
- PMID: 30700571
- PMCID: PMC6900432
- DOI: 10.1042/BSR20182345
Zinc binding regulates amyloid-like aggregation of GAPR-1
Abstract
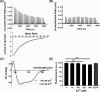
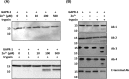
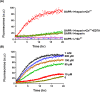
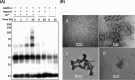
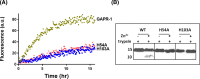
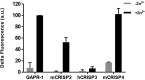
Members of the CAP superfamily (Cysteine-rich secretory proteins, Antigen 5, and Pathogenesis-related 1 proteins) are characterized by the presence of a CAP domain that is defined by four sequence motifs and a highly conserved tertiary structure. A common structure-function relationship for this domain is hitherto unknown. A characteristic of several CAP proteins is their formation of amyloid-like structures in the presence of lipids. Here we investigate the structural modulation of Golgi-Associated plant Pathogenesis Related protein 1 (GAPR-1) by known interactors of the CAP domain, preceding amyloid-like aggregation. Using isothermal titration calorimetry (ITC), we demonstrate that GAPR-1 binds zinc ions. Zn2+ binding causes a slight but significant conformational change as revealed by CD, tryptophan fluorescence, and trypsin digestion. The Zn2+-induced conformational change was required for the formation of GAPR-1 oligomers and amyloid-like assemblies in the presence of heparin, as shown by ThT fluorescence and TEM. Molecular dynamics simulations show binding of Zn2+ to His54 and His103 Mutation of these two highly conserved residues resulted in strongly diminished amyloid-like aggregation. Finally, we show that proteins from the cysteine-rich secretory protein (CRISP) subfamily are also able to form ThT-positive structures in vitro in a heparin- and Zn2+-dependent manner, suggesting that oligomerization regulated by metal ions could be a common structural property of the CAP domain.
Keywords: Amyloid; CAP superfamily; CRISP; GAPR-1; Heparin; Zinc.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Comment in
-
Cofactor-mediated amyloidogenesis.Biosci Rep. 2019 Mar 6;39(3):BSR20190327. doi: 10.1042/BSR20190327. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30814314 Free PMC article.
Similar articles
-
Metal ions and redox balance regulate distinct amyloid-like aggregation pathways of GAPR-1.Sci Rep. 2019 Oct 21;9(1):15048. doi: 10.1038/s41598-019-51232-7. Sci Rep. 2019. PMID: 31636315 Free PMC article.
-
Golgi-Associated plant Pathogenesis Related protein 1 (GAPR-1) forms amyloid-like fibrils by interaction with acidic phospholipids and inhibits Aβ aggregation.Amyloid. 2014 Jun;21(2):88-96. doi: 10.3109/13506129.2014.882304. Epub 2014 Jan 29. Amyloid. 2014. PMID: 24471790
-
Dynamic and Reversible Aggregation of the Human CAP Superfamily Member GAPR-1 in Protein Inclusions in Saccharomyces cerevisiae.J Mol Biol. 2021 Sep 17;433(19):167162. doi: 10.1016/j.jmb.2021.167162. Epub 2021 Jul 21. J Mol Biol. 2021. PMID: 34298062
-
Regulation of Functional Protein Aggregation by Multiple Factors: Implications for the Amyloidogenic Behavior of the CAP Superfamily Proteins.Int J Mol Sci. 2020 Sep 7;21(18):6530. doi: 10.3390/ijms21186530. Int J Mol Sci. 2020. PMID: 32906672 Free PMC article. Review.
-
The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense.Endocr Rev. 2008 Dec;29(7):865-97. doi: 10.1210/er.2008-0032. Epub 2008 Sep 29. Endocr Rev. 2008. PMID: 18824526 Review.
Cited by
-
Metal ions and redox balance regulate distinct amyloid-like aggregation pathways of GAPR-1.Sci Rep. 2019 Oct 21;9(1):15048. doi: 10.1038/s41598-019-51232-7. Sci Rep. 2019. PMID: 31636315 Free PMC article.
-
Cysteine-Rich Secretory Proteins (CRISP) are Key Players in Mammalian Fertilization and Fertility.Front Cell Dev Biol. 2021 Dec 14;9:800351. doi: 10.3389/fcell.2021.800351. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34970552 Free PMC article. Review.
-
Immunity functions of Arabidopsis pathogenesis-related 1 are coupled but not confined to its C-terminus processing and trafficking.Mol Plant Pathol. 2022 May;23(5):664-678. doi: 10.1111/mpp.13187. Epub 2022 Feb 4. Mol Plant Pathol. 2022. PMID: 35122385 Free PMC article.
-
Cofactor-mediated amyloidogenesis.Biosci Rep. 2019 Mar 6;39(3):BSR20190327. doi: 10.1042/BSR20190327. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30814314 Free PMC article.
-
The epididymis contributes to sperm DNA integrity and early embryo development through Cysteine-Rich Secretory Proteins.Elife. 2025 Apr 28;13:RP97105. doi: 10.7554/eLife.97105. Elife. 2025. PMID: 40293787 Free PMC article.
References
-
- Eberle H.B., Serrano R.L., Füllekrug J., Schlosser A., Lehmann W.D., Lottspeich F. et al. . (2002) Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J. Cell Sci. 115, 827–838 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

