Zinc binding regulates amyloid-like aggregation of GAPR-1
- PMID: 30700571
- PMCID: PMC6900432
- DOI: 10.1042/BSR20182345
Zinc binding regulates amyloid-like aggregation of GAPR-1
Abstract
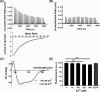
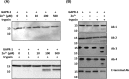
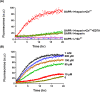
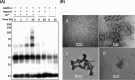
Members of the CAP superfamily (Cysteine-rich secretory proteins, Antigen 5, and Pathogenesis-related 1 proteins) are characterized by the presence of a CAP domain that is defined by four sequence motifs and a highly conserved tertiary structure. A common structure-function relationship for this domain is hitherto unknown. A characteristic of several CAP proteins is their formation of amyloid-like structures in the presence of lipids. Here we investigate the structural modulation of Golgi-Associated plant Pathogenesis Related protein 1 (GAPR-1) by known interactors of the CAP domain, preceding amyloid-like aggregation. Using isothermal titration calorimetry (ITC), we demonstrate that GAPR-1 binds zinc ions. Zn2+ binding causes a slight but significant conformational change as revealed by CD, tryptophan fluorescence, and trypsin digestion. The Zn2+-induced conformational change was required for the formation of GAPR-1 oligomers and amyloid-like assemblies in the presence of heparin, as shown by ThT fluorescence and TEM. Molecular dynamics simulations show binding of Zn2+ to His54 and His103 Mutation of these two highly conserved residues resulted in strongly diminished amyloid-like aggregation. Finally, we show that proteins from the cysteine-rich secretory protein (CRISP) subfamily are also able to form ThT-positive structures in vitro in a heparin- and Zn2+-dependent manner, suggesting that oligomerization regulated by metal ions could be a common structural property of the CAP domain.
Keywords: Amyloid; CAP superfamily; CRISP; GAPR-1; Heparin; Zinc.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

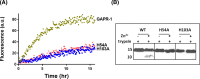
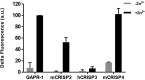
Comment in
-
Cofactor-mediated amyloidogenesis.Biosci Rep. 2019 Mar 6;39(3):BSR20190327. doi: 10.1042/BSR20190327. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30814314 Free PMC article.
References
-
- Eberle H.B., Serrano R.L., Füllekrug J., Schlosser A., Lehmann W.D., Lottspeich F. et al. (2002) Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J. Cell Sci. 115, 827–838 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

