FDG-PET patterns associated with underlying pathology in corticobasal syndrome
- PMID: 30700592
- PMCID: PMC6442013
- DOI: 10.1212/WNL.0000000000007038
FDG-PET patterns associated with underlying pathology in corticobasal syndrome
Abstract
Objective: To evaluate brain 18Fluorodeoxyglucose PET (FDG-PET) differences among patients with a clinical diagnosis of corticobasal syndrome (CBS) and distinct underling primary pathologies.
Methods: We studied 29 patients with a diagnosis of CBS who underwent FDG-PET scan and postmortem neuropathologic examination. Patients were divided into subgroups on the basis of primary pathologic diagnosis: CBS-corticobasal degeneration (CBS-CBD) (14 patients), CBS-Alzheimer disease (CBS-AD) (10 patients), and CBS-progressive supranuclear palsy (CBS-PSP) (5 patients). Thirteen age-matched healthy patients who underwent FDG-PET were the control group (HC). FDG-PET scans were compared between the subgroups and the HC using SPM-12, with a threshold of p FWE < 0.05.
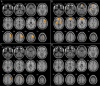
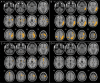
Results: There were no differences in Mattis Dementia Rating Scale or finger tapping scores between CBS groups. Compared to HC, the patients with CBS presented significant hypometabolism in frontoparietal regions, including the perirolandic area, basal ganglia, and thalamus of the clinically more affected hemisphere. Patients with CBS-CBD showed a similar pattern with a more marked, bilateral involvement of the basal ganglia. Patients with CBS-AD presented with posterior, asymmetric hypometabolism, including the lateral parietal and temporal lobes and the posterior cingulate. Finally, patients with CBS-PSP disclosed a more anterior hypometabolic pattern, including the medial frontal regions and the anterior cingulate. A conjunction analysis revealed that the primary motor cortex was the only common area of hypometabolism in all groups, irrespective of pathologic diagnosis.
Discussion and conclusions: In patients with CBS, different underling pathologies are associated with different patterns of hypometabolism. Our data suggest that FDG-PET scans could help in the etiologic diagnosis of CBS.
© 2019 American Academy of Neurology.
Figures

References
-
- Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 2012;83:405–410. - PubMed
-
- Boeve BF. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J Mol Neurosci 2011;45:350–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
