Rare Detection of Antiviral Functions of Polyclonal IgA Isolated from Plasma and Breast Milk Compartments in Women Chronically Infected with HIV-1
- PMID: 30700599
- PMCID: PMC6430545
- DOI: 10.1128/JVI.02084-18
Rare Detection of Antiviral Functions of Polyclonal IgA Isolated from Plasma and Breast Milk Compartments in Women Chronically Infected with HIV-1
Abstract
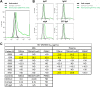
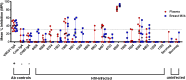
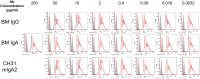
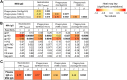
The humoral response to invading mucosal pathogens comprises multiple antibody isotypes derived from systemic and mucosal compartments. To understand the contribution of each antibody isotype/source to the mucosal humoral response, parallel investigation of the specificities and functions of antibodies within and across isotypes and compartments is required. The role of IgA against HIV-1 is complex, with studies supporting a protective role as well as a role for serum IgA in blocking effector functions. Thus, we explored the fine specificity and function of IgA in both plasma and mucosal secretions important to infant HIV-1 infection, i.e., breast milk. IgA and IgG were isolated from milk and plasma from 20 HIV-1-infected lactating Malawian women. HIV-1 binding specificities, neutralization potency, inhibition of virus-epithelial cell binding, and antibody-mediated phagocytosis were measured. Fine-specificity mapping showed IgA and IgG responses to multiple HIV-1 Env epitopes, including conformational V1/V2 and linear V2, V3, and constant region 5 (C5). Env IgA was heterogeneous between the milk and systemic compartments (Env IgA, τ = 0.00 to 0.63, P = 0.0046 to 1.00). Furthermore, IgA and IgG appeared compartmentalized as there was a lack of correlation between the specificities of Env-specific IgA and IgG (in milk, τ = -0.07 to 0.26, P = 0.35 to 0.83). IgA and IgG also differed in functions: while neutralization and phagocytosis were consistently mediated by milk and plasma IgG, they were rarely detected in IgA from both milk and plasma. Understanding the ontogeny of the divergent IgG and IgA antigen specificity repertoires and their effects on antibody function will inform vaccination approaches targeted toward mucosal pathogens.IMPORTANCE Antibodies within the mucosa are part of the first line of defense against mucosal pathogens. Evaluating mucosal antibody isotypes, specificities, and antiviral functions in relationship to the systemic antibody profile can provide insights into whether the antibody response is coordinated in response to mucosal pathogens. In a natural immunity cohort of HIV-infected lactating women, we mapped the fine specificity and function of IgA in breast milk and plasma and compared these with the autologous IgG responses. Antigen specificities and functions differed between IgG and IgA, with antiviral functions (neutralization and phagocytosis) predominantly mediated by the IgG fraction in both milk and plasma. Furthermore, the specificity of milk IgA differed from that of systemic IgA. Our data suggest that milk IgA and systemic IgA should be separately examined as potential correlates of risk. Preventive vaccines may need to employ different strategies to elicit functional antiviral immunity by both antibody isotypes in the mucosa.
Keywords: HIV-1; IgA; effector functions; mucosal immunity.
Copyright © 2019 Tay et al.
Figures







Similar articles
-
Combined HIV-1 Envelope Systemic and Mucosal Immunization of Lactating Rhesus Monkeys Induces a Robust Immunoglobulin A Isotype B Cell Response in Breast Milk.J Virol. 2016 Apr 29;90(10):4951-4965. doi: 10.1128/JVI.00335-16. Print 2016 May 15. J Virol. 2016. PMID: 26937027 Free PMC article.
-
HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies.J Virol. 2011 Sep;85(18):9555-67. doi: 10.1128/JVI.05174-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734046 Free PMC article.
-
Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk.J Virol. 2013 Jun;87(12):6986-99. doi: 10.1128/JVI.00528-13. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596289 Free PMC article.
-
Nonneutralizing functional antibodies: a new "old" paradigm for HIV vaccines.Clin Vaccine Immunol. 2014 Aug;21(8):1023-36. doi: 10.1128/CVI.00230-14. Epub 2014 Jun 11. Clin Vaccine Immunol. 2014. PMID: 24920599 Free PMC article. Review.
-
Humoral immune responses to HIV in the mucosal secretions and sera of HIV-infected women.Am J Reprod Immunol. 2014 Jun;71(6):600-7. doi: 10.1111/aji.12203. Epub 2014 Feb 5. Am J Reprod Immunol. 2014. PMID: 24494997 Free PMC article. Review.
Cited by
-
Unique Kinetics of the Human Milk Antibody Response to JYNNEOS Vaccine for Prevention of Monkey Pox: A Case Study.Breastfeed Med. 2024 Dec;19(12):974-979. doi: 10.1089/bfm.2024.0257. Epub 2024 Oct 2. Breastfeed Med. 2024. PMID: 39360771
-
Monocyte-derived transcriptome signature indicates antibody-dependent cellular phagocytosis as a potential mechanism of vaccine-induced protection against HIV-1.Elife. 2021 Sep 17;10:e69577. doi: 10.7554/eLife.69577. Elife. 2021. PMID: 34533134 Free PMC article.
-
Polyclonal Broadly Neutralizing Antibody Activity Characterized by CD4 Binding Site and V3-Glycan Antibodies in a Subset of HIV-1 Virus Controllers.Front Immunol. 2021 Dec 23;12:670561. doi: 10.3389/fimmu.2021.670561. eCollection 2021. Front Immunol. 2021. PMID: 35003053 Free PMC article.
-
IgA Summons IgG to Take a Hit at HIV-1.Cell Host Microbe. 2020 Jun 10;27(6):854-856. doi: 10.1016/j.chom.2020.05.017. Cell Host Microbe. 2020. PMID: 32526180 Free PMC article.
-
Vertical HIV-1 Transmission in the Setting of Maternal Broad and Potent Antibody Responses.J Virol. 2022 Jun 8;96(11):e0023122. doi: 10.1128/jvi.00231-22. Epub 2022 May 10. J Virol. 2022. PMID: 35536018 Free PMC article.
References
-
- Besredka A. 1927. Local immunization. Bailliere, Tindall Cox, London, UK.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

