Flexible decapyrrylcorannulene hosts
- PMID: 30700716
- PMCID: PMC6353959
- DOI: 10.1038/s41467-019-08343-6
Flexible decapyrrylcorannulene hosts
Abstract
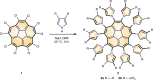
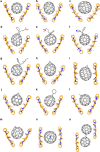
The assembly of spherical fullerenes, or buckyballs, into single crystals for crystallographic identification often suffers from disordered arrangement. Here we show a chiral configuration of decapyrrylcorannulene that has a concave 'palm' of corannulene and ten flexible electron-rich pyrryl group 'fingers' to mimic the smart molecular 'hands' for self-adaptably cradling various buckyballs in a (+)hand-ball-hand(-) mode. As exemplified by crystallographic identification of 15 buckyball structures representing pristine, exohedral, endohedral, dimeric and hetero-derivatization, the pyrryl groups twist with varying dihedral angles to adjust the interaction between decapyrrylcorannulene and fullerene. The self-adaptable electron-rich pyrryl groups, susceptible to methylation, are theoretically revealed to contribute more than the bowl-shaped palm of the corannulene in holding buckyball structures. The generality of the present decapyrrylcorannulene host with flexible pyrryl groups facilitates the visualization of numerous unknown/unsolved fullerenes by crystallography and the assembly of the otherwise close-packed spherical fullerenes into two-dimensional layered structures by intercalation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

