Progesterone Treatment Attenuates Glycolytic Metabolism and Induces Senescence in Glioblastoma
- PMID: 30700763
- PMCID: PMC6353890
- DOI: 10.1038/s41598-018-37399-5
Progesterone Treatment Attenuates Glycolytic Metabolism and Induces Senescence in Glioblastoma
Abstract
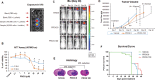
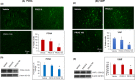
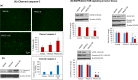
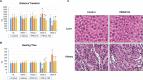
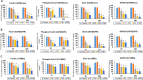
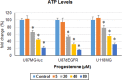
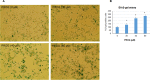
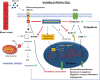
We examined the effect of progesterone treatments on glycolytic metabolism and senescence as possible mechanisms in controlling the growth of glioblastoma multiforme (GBM). In an orthotopic mouse model, after tumor establishment, athymic nude mice received treatment with progesterone or vehicle for 40 days. Compared to controls, high-dose progesterone administration produced a significant reduction in tumor size (~47%) and an increased survival rate (~43%) without any demonstrable toxicity to peripheral organs (liver, kidney). This was accompanied by a significant improvement in spontaneous locomotor activity and reduced anxiety-like behavior. In a follow-up in vitro study of U87MG-luc, U87dEGFR and U118MG tumor cells, we observed that high-dose progesterone inhibited expression of Glut1, which facilitated glucose transport into the cytoplasm; glyceraldehyde 3-phosphate dehydrogenase (GAPDH; a glycolysis enzyme); ATP levels; and cytoplasmic FoxO1 and Phospho-FoxO1, both of which control glycolytic metabolism through upstream PI3K/Akt/mTOR signaling in GBM. In addition, progesterone administration attenuated EGFR/PI3K/Akt/mTOR signaling, which is highly activated in grade IV GBM. High-dose progesterone also induced senescence in GBM as evidenced by changes in cell morphology and β-galactocidase accumulation. In conclusion, progesterone inhibits the modulators of glycolytic metabolism and induces premature senescence in GBM cells and this can help to reduce/slow tumor progression.
Conflict of interest statement
A US patent (#US 8,435,972 B2) was issued to FA and DGS on May 7, 2013 for the use of progesterone and compositions related thereto.
Figures

References
-
- American Brain Tumor Association. American Brain Tumor Association Brain Tumor Statistics. (Chicago, IL, 2014). http://abta.pub30.convio.net/about-us/news/brain-tumor-statistics/.
-
- Central Brain Tumor Registry of the United States. CBTRUS Staistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. (CBTRUS, Hinsdale, Illinois, 2010).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

