Creation of haemoglobin A1c direct oxidase from fructosyl peptide oxidase by combined structure-based site specific mutagenesis and random mutagenesis
- PMID: 30700768
- PMCID: PMC6353924
- DOI: 10.1038/s41598-018-37806-x
Creation of haemoglobin A1c direct oxidase from fructosyl peptide oxidase by combined structure-based site specific mutagenesis and random mutagenesis
Abstract
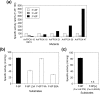
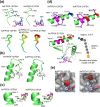
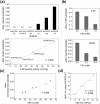
The currently available haemoglobin A1c (HbA1c) enzymatic assay consists of two specific steps: proteolysis of HbA1c and oxidation of the liberated fructosyl peptide by fructosyl peptide oxidase (FPOX). To develop a more convenient and high throughput assay, we devised novel protease-free assay system employing modified FPOX with HbA1c oxidation activity, namely HbA1c direct oxidase (HbA1cOX). AnFPOX-15, a modified FPOX from Aspergillus nidulans, was selected for conversion to HbA1cOX. As deduced from the crystal structure of AnFPOX-15, R61 was expected to obstruct the entrance of bulky substrates. An R61G mutant was thus constructed to open the gate at the active site. The prepared mutant exhibited significant reactivity for fructosyl hexapeptide (F-6P, N-terminal amino acids of HbA1c), and its crystal structure revealed a wider gate observed for AnFPOX-15. To improve the reactivity for F-6P, several mutagenesis approaches were performed. The ultimately generated AnFPOX-47 exhibited the highest F-6P reactivity and possessed HbA1c oxidation activity. HbA1c levels in blood samples as measured using the direct assay system using AnFPOX-47 were highly correlated with the levels measured using the conventional HPLC method. In this study, FPOX was successfully converted to HbA1cOX, which could represent a novel in vitro diagnostic modality for diabetes mellitus.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Engineering an efficient mutant of Eupenicillium terrenum fructosyl peptide oxidase for the specific determination of hemoglobin A1c.Appl Microbiol Biotechnol. 2019 Feb;103(4):1725-1735. doi: 10.1007/s00253-018-9529-9. Epub 2019 Jan 3. Appl Microbiol Biotechnol. 2019. PMID: 30607487
-
Engineering fructosyl peptide oxidase to improve activity toward the fructosyl hexapeptide standard for HbA1c measurement.Mol Biotechnol. 2013 Jul;54(3):939-43. doi: 10.1007/s12033-012-9644-2. Mol Biotechnol. 2013. PMID: 23335126
-
A comprehensive review on fructosyl peptide oxidase as an important enzyme for present hemoglobin A1c assays.Biotechnol Appl Biochem. 2025 Feb;72(1):268-281. doi: 10.1002/bab.2647. Epub 2024 Aug 4. Biotechnol Appl Biochem. 2025. PMID: 39099239 Review.
-
An amperometric biosensor for specific detection of glycated hemoglobin based on recombinant engineered fructosyl peptide oxidase.Int J Biol Macromol. 2020 Jan 1;142:855-865. doi: 10.1016/j.ijbiomac.2019.10.025. Epub 2019 Oct 14. Int J Biol Macromol. 2020. PMID: 31622711
-
Review of fructosyl amino acid oxidase engineering research: a glimpse into the future of hemoglobin A1c biosensing.J Diabetes Sci Technol. 2009 May 1;3(3):585-92. doi: 10.1177/193229680900300324. J Diabetes Sci Technol. 2009. PMID: 20144298 Free PMC article. Review.
Cited by
-
Direct glucosone-based synthesis and HILIC-ESI-MS/MS characterization of N-terminal fructosylated valine and valylhistidine for validation of enzymatic HbA1c assays in the diagnosis of diabetes mellitus.Anal Bioanal Chem. 2019 Dec;411(30):7967-7979. doi: 10.1007/s00216-019-02186-2. Epub 2019 Nov 22. Anal Bioanal Chem. 2019. PMID: 31754770 Free PMC article.
-
A Review of Electrochemical Sensors for the Detection of Glycated Hemoglobin.Biosensors (Basel). 2022 Apr 8;12(4):221. doi: 10.3390/bios12040221. Biosensors (Basel). 2022. PMID: 35448281 Free PMC article. Review.
-
Tailoring FPOX enzymes for enhanced stability and expanded substrate recognition.Sci Rep. 2023 Oct 30;13(1):18610. doi: 10.1038/s41598-023-45428-1. Sci Rep. 2023. PMID: 37903872 Free PMC article.
-
Detection of Biomarker Using Aptasensors to Determine the Type of Diabetes.Diagnostics (Basel). 2023 Jun 12;13(12):2035. doi: 10.3390/diagnostics13122035. Diagnostics (Basel). 2023. PMID: 37370930 Free PMC article. Review.
-
In Silico Engineering of Enzyme Access Tunnels.Methods Mol Biol. 2022;2397:203-225. doi: 10.1007/978-1-0716-1826-4_11. Methods Mol Biol. 2022. PMID: 34813066
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

