Genome-wide association study of antidepressant treatment resistance in a population-based cohort using health service prescription data and meta-analysis with GENDEP
- PMID: 30700811
- PMCID: PMC7096334
- DOI: 10.1038/s41397-019-0067-3
Genome-wide association study of antidepressant treatment resistance in a population-based cohort using health service prescription data and meta-analysis with GENDEP
Abstract
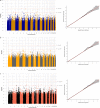
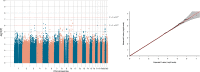
Antidepressants demonstrate modest response rates in the treatment of major depressive disorder (MDD). Despite previous genome-wide association studies (GWAS) of antidepressant treatment response, the underlying genetic factors are unknown. Using prescription data in a population and family-based cohort (Generation Scotland: Scottish Family Health Study; GS:SFHS), we sought to define a measure of (a) antidepressant treatment resistance and (b) stages of antidepressant resistance by inferring antidepressant switching as non-response to treatment. GWAS were conducted separately for antidepressant treatment resistance in GS:SFHS and the Genome-based Therapeutic Drugs for Depression (GENDEP) study and then meta-analysed (meta-analysis n = 4213, cases = 358). For stages of antidepressant resistance, a GWAS on GS:SFHS only was performed (n = 3452). Additionally, we conducted gene-set enrichment, polygenic risk scoring (PRS) and genetic correlation analysis. We did not identify any significant loci, genes or gene sets associated with antidepressant treatment resistance or stages of resistance. Significant positive genetic correlations of antidepressant treatment resistance and stages of resistance with neuroticism, psychological distress, schizotypy and mood disorder traits were identified. These findings suggest that larger sample sizes are needed to identify the genetic architecture of antidepressant treatment response, and that population-based observational studies may provide a tractable approach to achieving the necessary statistical power.
Conflict of interest statement
AMM has received financial support from Pfizer (formerly Wyeth), Janssen and Lilly and from the Sackler trust. The remaining authors declare that they have no conflict of interest.
Figures
References
-
- Ustün T, Ayuso-Mateos J, Chatterji S, Mathers C, Murray C. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. - PubMed
-
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. - PMC - PubMed
-
- Rush A, Trivedi M, Wisniewski S, Nierenberg A, Stewart J, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. - PubMed
-
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

