Knockout of α-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes
- PMID: 30700848
- PMCID: PMC6570540
- DOI: 10.1038/s41374-018-0178-5
Knockout of α-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes
Erratum in
-
Correction to: Knockout of α-calcitonin gene-related peptide attenuates cholestatic liver injury by differentially regulating cellular senescence of hepatic stellate cells and cholangiocytes.Lab Invest. 2020 May;100(5):788. doi: 10.1038/s41374-020-0376-9. Lab Invest. 2020. PMID: 31937871
Abstract
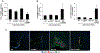
α-Calcitonin gene-related peptide (α-CGRP) is a 37-amino acid neuropeptide involved in several pathophysiological processes. α-CGRP is involved in the regulation of cholangiocyte proliferation during cholestasis. In this study, we aimed to evaluate if α-CGRP regulates bile duct ligation (BDL)-induced liver fibrosis by using a α-CGRP knockout (α-CGRP-/-) mouse model. α-CGRP-/- and wild-type (WT) mice were subjected to sham surgery or BDL for 7 days. Then, liver fibrosis and cellular senescence as well as the expression of kinase such as p38 and C-Jun N-terminal protein kinase (JNK) in mitogen-activated protein kinases (MAPK) signaling pathway were evaluated in total liver, together with measurement of cellular senescence in cholangiocytes or hepatic stellate cells (HSCs). There was enhanced hepatic expression of Calca (coding α-CGRP) and the CGRP receptor components (CRLR, RAMP-1 and RCP) in BDL and in both WT α-CGRP-/- and BDL α-CGRP-/- mice, respectively. Moreover, there was increased CGRP serum levels and hepatic mRNA expression of CALCA and CGRP receptor components in late-stage PSC samples compared to healthy control samples. Depletion of α-CGRP reduced liver injury and fibrosis in BDL mice that was associated with enhanced cellular senescence of hepatic stellate cells and reduced senescence of cholangiocytes as well as decreased activation of p38 and JNK MAPK signaling pathway. Cholangiocyte supernatant from BDL α-CGRP-/- mice inhibited the activation and increased cellular senescence of cultured human HSCs (HHSCs) compared to HHSCs stimulated with BDL cholangiocyte supernatant. Taken together, endogenous α-CGRP promoted BDL-induced cholestatic liver fibrosis through differential changes in senescence of HSCs and cholangiocytes and activation of p38 and JNK signaling. Modulation of α-CGRP/CGRP receptor signaling may be key for the management of biliary senescence and liver fibrosis in cholangiopathies.
Figures
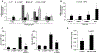


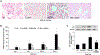



Similar articles
-
Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice.Lab Invest. 2007 Sep;87(9):914-26. doi: 10.1038/labinvest.3700602. Epub 2007 Jul 9. Lab Invest. 2007. PMID: 17618297
-
Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells.Hepatology. 2017 Aug;66(2):528-541. doi: 10.1002/hep.29138. Epub 2017 Jun 19. Hepatology. 2017. PMID: 28256736 Free PMC article.
-
Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis.Hepatology. 2019 Oct;70(4):1317-1335. doi: 10.1002/hep.30662. Epub 2019 May 24. Hepatology. 2019. PMID: 30985008 Free PMC article.
-
Cellular senescence in the cholangiopathies.Curr Opin Gastroenterol. 2022 Mar 1;38(2):121-127. doi: 10.1097/MOG.0000000000000805. Curr Opin Gastroenterol. 2022. PMID: 35098933 Free PMC article. Review.
-
Fibrotic Events in the Progression of Cholestatic Liver Disease.Cells. 2021 May 5;10(5):1107. doi: 10.3390/cells10051107. Cells. 2021. PMID: 34062960 Free PMC article. Review.
Cited by
-
Mapping the evolution of liver aging research: A bibliometric analysis.World J Gastroenterol. 2024 Nov 7;30(41):4461-4480. doi: 10.3748/wjg.v30.i41.4461. World J Gastroenterol. 2024. PMID: 39534417 Free PMC article.
-
Knockdown of vimentin reduces mesenchymal phenotype of cholangiocytes in the Mdr2-/- mouse model of primary sclerosing cholangitis (PSC).EBioMedicine. 2019 Oct;48:130-142. doi: 10.1016/j.ebiom.2019.09.013. Epub 2019 Sep 12. EBioMedicine. 2019. PMID: 31522982 Free PMC article.
-
Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell-Derived Transforming Growth Factor Beta 1 Signaling.Hepatology. 2021 Jun;73(6):2397-2410. doi: 10.1002/hep.31497. Epub 2021 Apr 19. Hepatology. 2021. Retraction in: Hepatology. 2025 May 1;81(5):E153. doi: 10.1097/HEP.0000000000000798. PMID: 32761972 Free PMC article. Retracted.
-
Current Perspectives of Neuroendocrine Regulation in Liver Fibrosis.Cells. 2022 Nov 26;11(23):3783. doi: 10.3390/cells11233783. Cells. 2022. PMID: 36497043 Free PMC article. Review.
-
The Effects of Taurocholic Acid on Biliary Damage and Liver Fibrosis Are Mediated by Calcitonin-Gene-Related Peptide Signaling.Cells. 2022 May 9;11(9):1591. doi: 10.3390/cells11091591. Cells. 2022. PMID: 35563897 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108959/DK/NIDDK NIH HHS/United States
- R21 AA025157/AA/NIAAA NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- R01 DK058411/DK/NIDDK NIH HHS/United States
- R01 DK107310/DK/NIDDK NIH HHS/United States
- I01 BX003031/BX/BLRD VA/United States
- IK6 BX004601/BX/BLRD VA/United States
- R21 AA025997/AA/NIAAA NIH HHS/United States
- R01 DK110035/DK/NIDDK NIH HHS/United States
- R01 DK054811/DK/NIDDK NIH HHS/United States
- I01 BX000574/BX/BLRD VA/United States
- I01 BX001724/BX/BLRD VA/United States
- R01 DK076898/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

