T cell antigen discovery via trogocytosis
- PMID: 30700903
- PMCID: PMC6719556
- DOI: 10.1038/s41592-018-0305-7
T cell antigen discovery via trogocytosis
Abstract
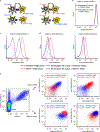
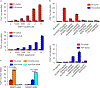
T cell receptor (TCR) ligand discovery is essential for understanding and manipulating immune responses to tumors. We developed a cell-based selection platform for TCR ligand discovery that exploits a membrane transfer phenomenon called trogocytosis. We discovered that T cell membrane proteins are transferred specifically to target cells that present cognate peptide-major histocompatibility complex (MHC) molecules. Co-incubation of T cells expressing an orphan TCR with target cells collectively presenting a library of peptide-MHCs led to specific labeling of cognate target cells, enabling isolation of these target cells and sequencing of the cognate TCR ligand. We validated this method for two clinically employed TCRs and further used the platform to identify the cognate neoepitope for a subject-derived neoantigen-specific TCR. Thus, target cell trogocytosis is a robust tool for TCR ligand discovery that will be useful for studying basic tumor immunology and identifying new targets for immunotherapy.
Figures



References
-
- Dembic Z et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature 320, 232–238 (1986). - PubMed
-
- Schumacher TN & Schreiber RD Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). - PubMed
-
- Sollid LM et al. Small bowel, celiac disease and adaptive immunity. Dig. Dis. 33, 115–121 (2015). - PubMed
-
- Kontos S, Grimm AJ & Hubbell JA Engineering antigen-specific immunological tolerance. Curr. Opin. Immunol. 35, 80–88 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

