The palladacycle complex AJ-5 induces apoptotic cell death while reducing autophagic flux in rhabdomyosarcoma cells
- PMID: 30701092
- PMCID: PMC6349869
- DOI: 10.1038/s41420-019-0139-9
The palladacycle complex AJ-5 induces apoptotic cell death while reducing autophagic flux in rhabdomyosarcoma cells
Abstract
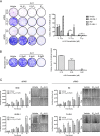
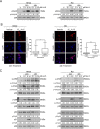
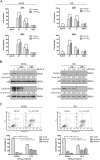
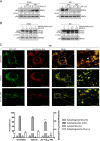
Rhabdomyosarcoma (RMS) forms in skeletal muscle and is the most common soft tissue sarcoma in children and adolescents. Current treatment is associated with debilitating side effects and treatment outcomes for patients with metastatic disease are dismal. Recently, a novel binuclear palladacycle, AJ-5, was shown to exert potent cytotoxicity in melanoma and breast cancer and to present with negligible adverse effects in mice. This study investigates the anti-cancer activity of AJ-5 in alveolar and embryonal RMS. IC50 values of ≤ 0.2 µM were determined for AJ-5 and it displayed a favourable selectivity index of >2. Clonogenic and migration assays showed that AJ-5 inhibited the ability of RMS cells to survive and migrate, respectively. Western blotting revealed that AJ-5 induced levels of key DNA damage response proteins (γH2AX, p-ATM and p-Chk2) and the p38/MAPK stress pathway. This correlated with an upregulation of p21 and a G1 cell cycle arrest. Annexin V-FITC/propidium iodide staining revealed that AJ-5 induced apoptosis and necrosis. Apoptosis was confirmed by the detection of cleaved PARP and increased levels and activity of cleaved caspases-3, -7, -8 and -9. Furthermore, AJ-5 reduced autophagic flux as shown by reduced LC3II accumulation in the presence of bafilomycin A1 and a significant reduction in autophagosome flux J. Finally, pharmacokinetic studies in mice show that AJ-5 has a promising half-life and that its volume of distribution is high, its clearance low and its intraperitoneal absorption is good. Together these findings suggest that AJ-5 may be an effective chemotherapeutic with a desirable mechanism of action for treating drug-resistant and advanced sarcomas.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

