Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations
- PMID: 30701144
- PMCID: PMC6344032
- DOI: 10.1021/acssuschemeng.8b05832
Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations
Abstract
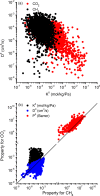
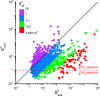
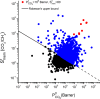
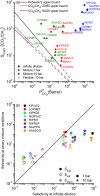
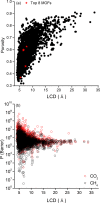
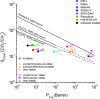
Efficient separation of CO2 from CO2/CH4 mixtures using membranes has economic, environmental and industrial importance. Membrane technologies are currently dominated by polymers due to their processing abilities and low manufacturing costs. However, polymeric membranes suffer from either low gas permeabilities or low selectivities. Metal organic frameworks (MOFs) are suggested as potential membrane candidates that offer both high selectivity and permeability for CO2/CH4 separation. Experimental testing of every single synthesized MOF material as membranes is not practical due to the availability of thousands of different MOF materials. A multilevel, high-throughput computational screening methodology was used to examine the MOF database for membrane-based CO2/CH4 separation. MOF membranes offering the best combination of CO2 permeability (>106 Barrer) and CO2/CH4 selectivity (>80) were identified by combining grand canonical Monte Carlo and molecular dynamics simulations. Results revealed that the best MOF membranes are located above the Robeson's upper bound indicating that they outperform polymeric membranes for CO2/CH4 separation. The impact of framework flexibility on the membrane properties of the selected top MOFs was studied by comparing the results of rigid and flexible molecular simulations. Relations between structures and performances of MOFs were also investigated to provide atomic-level insights into the design of novel MOFs which will be useful for CO2/CH4 separation processes. We also predicted permeabilities and selectivities of the mixed matrix membranes (MMM) in which the best MOF candidates are incorporated as filler particles into polymers and found that MOF-based MMMs have significantly higher CO2 permeabilities and moderately higher selectivities than pure polymers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Guo A.; Ban Y. J.; Yang K.; Yang W. S. Metal-organic framework-based mixed matrix membranes: Synergetic effect of adsorption and diffusion for CO2/CH4 separation. J. Membr. Sci. 2018, 562, 76–84. 10.1016/j.memsci.2018.05.032. - DOI
-
- Zhang Y.; Sunarso J.; Liu S. M.; Wang R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenhouse Gas Control 2013, 12, 84–107. 10.1016/j.ijggc.2012.10.009. - DOI
-
- Venna S. R.; Carreon M. A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. 10.1016/j.ces.2014.10.007. - DOI
-
- Li S.; Falconer J. L.; Noble R. D. Improved SAPO-34 membranes for CO2/CH4 separations. Adv. Mater. 2006, 18, 2601–2603. 10.1002/adma.200601147. - DOI
-
- Falconer J. L.; Carreon M. A.; Li S.; Noble R. D.. Synthesis of zeolites and zeolite membranes using multiple structure directing agents. U.S. Patent 8,302,782, 2012.
LinkOut - more resources
Full Text Sources
