Molecular cloning and transcriptional regulation of Indian peafowl (Pavo cristatus) IFN-α gene
- PMID: 30701479
- PMCID: PMC6439081
- DOI: 10.1007/s12192-018-00962-0
Molecular cloning and transcriptional regulation of Indian peafowl (Pavo cristatus) IFN-α gene
Abstract
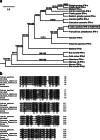
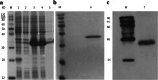
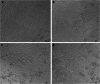
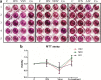
Interferon-α (IFN-α) resists viral infections by triggering the transcription of a diverse range of antiviral IFN-stimulated genes (ISGs). However, information about the Indian peafowl (Pavo cristatus) IFN-α (PcIFN-α) has not been reported. In this study, a PcIFN-α gene was amplified, which encoded a protein of 193 amino acids with a 26-amino acid signal peptide sharing 72.16-95.70% identity with other avians in Aves. After expression in prokaryote, PcIFN-α was analyzed for its physicochemical property and antiviral activity. Intriguingly, compared with chicken IFN-α, an effective viral infection therapeutic agent, PcIFN-α showed superior anti-VSV, NDV, and AIV activities, which were then abrogated by rabbit anti-PcIFN-α antibodies in vitro. Moreover, PcIFN-α was shown to be highly sensitive to trypsin; however, it remained stable despite changes in pH and temperature. Additionally, PcIFN-α induced the transcriptional or translational levels of Mx1 and ISG12 genes time-dependently. Overall, the present study revealed that PcIFN-α is a potential novel effective therapeutic agent in antiviral defense responses in peafowl, improving understanding of its involvement in bird antiviral defense.
Keywords: Characterization; Interferon-stimulated genes; Interferon-α; Molecular cloning; Pavo cristatus.
Figures


References
-
- Alexander DJ. Paramyxoviridae (Newcastle disease and others), Vol. London, UK: W.B. Saunders; 1996.
-
- Dou FM, Yang WJ (2007) Isolation and identification of NDV from peacock. J Econ Anim 11:9
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

