Visualization of currents in neural models with similar behavior and different conductance densities
- PMID: 30702427
- PMCID: PMC6395073
- DOI: 10.7554/eLife.42722
Visualization of currents in neural models with similar behavior and different conductance densities
Abstract
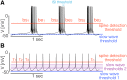
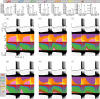
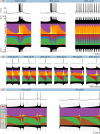
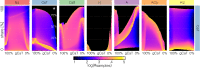
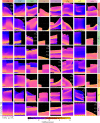
Conductance-based models of neural activity produce large amounts of data that can be hard to visualize and interpret. We introduce visualization methods to display the dynamics of the ionic currents and to display the models' response to perturbations. To visualize the currents' dynamics, we compute the percent contribution of each current and display them over time using stacked-area plots. The waveform of the membrane potential and the contribution of each current change as the models are perturbed. To represent these changes over a range of the perturbation control parameter, we compute and display the distributions of these waveforms. We illustrate these procedures in six examples of bursting model neurons with similar activity but that differ as much as threefold in their conductance densities. These visualization methods provide heuristic insight into why individual neurons or networks with similar behavior can respond widely differently to perturbations.
Keywords: Ca++ channels; K+ channels; Na+ channels; conductance-based; ionic channels; neuronal oscillators; neuroscience; none.
© 2019, Alonso and Marder.
Conflict of interest statement
LA No competing interests declared, EM Senior editor, eLife
Figures











References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

