Oea Signaling Pathways and the Metabolic Benefits of Vertical Sleeve Gastrectomy
- PMID: 30702457
- PMCID: PMC7451489
- DOI: 10.1097/SLA.0000000000003093
Oea Signaling Pathways and the Metabolic Benefits of Vertical Sleeve Gastrectomy
Abstract
Objective: The aim of this study was to determine whether downstream [peroxisome proliferator-activated-receptor alpha (PPARα) and the G-protein coupled receptor, GPR119] and upstream (a fatty acid translocase, CD36) signaling targets of N-oleoylethanolamide (OEA) were necessary for weight loss, metabolic improvements, and diet preference following vertical sleeve gastrectomy (VSG).
Summary background data: OEA is an anorectic N-acylethanolamine produced from dietary fats within the intestinal lumen that can modulate lipid metabolism, insulin secretion, and energy expenditure by activating targets such as PPARα and GPR119.
Methods: Diet-induced obese mice, including wild-type or whole body knockout (KO) of PPARα, GPR119, and CD36, were stratified to either VSG or sham surgery before body weight, body composition, diet preference, and glucose and lipid metabolic endpoints were assessed.
Results: We found increased duodenal production of OEA and expression of both GPR119 and CD36 were upregulated in wild-type mice after VSG. However, weight loss and glucose tolerance were improved in response to VSG in PPARαKO, GPR119KO, and CD36KO mice. In fact, VSG corrected hepatic triglyceride dysregulation in CD36KO mice, and circulating triglyceride and cholesterol levels in PPARαKO mice. Lastly, we found PPARα-mediated signaling contributes to macronutrient preference independent of VSG, while removal of CD36 signaling blunts the VSG-induced shift toward carbohydrate preference.
Conclusions: In the search for more effective and less invasive therapies to help reverse the global acceleration of obesity and obesity-related disease OEA is a promising candidate; however, our data indicate that it is not an underlying mechanism of the effectiveness of VSG.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
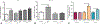

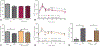
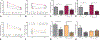
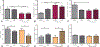

Similar articles
-
TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice.Gut. 2017 Feb;66(2):226-234. doi: 10.1136/gutjnl-2015-309871. Epub 2015 Oct 28. Gut. 2017. PMID: 26511794 Free PMC article.
-
Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion.Gastroenterology. 2011 Sep;141(3):939-949.e1-4. doi: 10.1053/j.gastro.2011.05.008. Epub 2011 May 18. Gastroenterology. 2011. PMID: 21699773 Free PMC article.
-
Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats.Neuropharmacology. 2005 Jun;48(8):1147-53. doi: 10.1016/j.neuropharm.2005.02.013. Epub 2005 Apr 21. Neuropharmacology. 2005. PMID: 15910890
-
A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-α, on the management and prevention of obesity.Clin Exp Pharmacol Physiol. 2020 Apr;47(4):543-552. doi: 10.1111/1440-1681.13238. Epub 2020 Jan 31. Clin Exp Pharmacol Physiol. 2020. PMID: 31868943
-
Oleic acid-derived oleoylethanolamide: A nutritional science perspective.Prog Lipid Res. 2017 Jul;67:1-15. doi: 10.1016/j.plipres.2017.04.001. Epub 2017 Apr 5. Prog Lipid Res. 2017. PMID: 28389247 Review.
Cited by
-
The effect of bariatric surgery on the expression of gastrointestinal taste receptors: A systematic review.Rev Endocr Metab Disord. 2024 Apr;25(2):421-446. doi: 10.1007/s11154-023-09865-7. Epub 2024 Jan 11. Rev Endocr Metab Disord. 2024. PMID: 38206483 Free PMC article.
-
Glycemic effect of pancreatic preproglucagon in mouse sleeve gastrectomy.JCI Insight. 2019 Oct 17;4(20):e129452. doi: 10.1172/jci.insight.129452. JCI Insight. 2019. PMID: 31619587 Free PMC article.
-
Diet-dependent sex differences in the response to vertical sleeve gastrectomy.Am J Physiol Endocrinol Metab. 2021 Jul 1;321(1):E11-E23. doi: 10.1152/ajpendo.00060.2021. Epub 2021 May 17. Am J Physiol Endocrinol Metab. 2021. PMID: 33998293 Free PMC article.
-
Peroxisome Proliferator-Activated Receptor-α: A Pivotal Regulator of the Gastrointestinal Tract.Front Mol Biosci. 2022 Apr 26;9:864039. doi: 10.3389/fmolb.2022.864039. eCollection 2022. Front Mol Biosci. 2022. PMID: 35558563 Free PMC article. Review.
-
Hypoabsorptive surgeries cause limb-dependent changes in the gut endocannabinoidome and microbiome in association with beneficial metabolic effects.Int J Obes (Lond). 2023 Jul;47(7):630-641. doi: 10.1038/s41366-023-01307-3. Epub 2023 May 4. Int J Obes (Lond). 2023. PMID: 37142736
References
-
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016 Key findings Data from the National Health and Nutrition Examination Survey. Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf 2015. Accessed November 10, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

