Vagal afferents contribute to sympathoexcitation-driven metabolic dysfunctions
- PMID: 30703063
- PMCID: PMC6368248
- DOI: 10.1530/JOE-18-0623
Vagal afferents contribute to sympathoexcitation-driven metabolic dysfunctions
Abstract
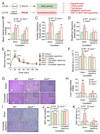
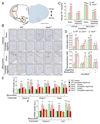
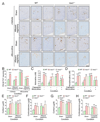
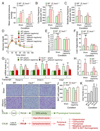
Multiple crosstalk between peripheral organs and the nervous system are required to maintain physiological and metabolic homeostasis. Using Vav3-deficient mice as a model for chronic sympathoexcitation-associated disorders, we report here that afferent fibers of the hepatic branch of the vagus nerve are needed for the development of the peripheral sympathoexcitation, tachycardia, tachypnea, insulin resistance, liver steatosis and adipose tissue thermogenesis present in those mice. This neuronal pathway contributes to proper activity of the rostral ventrolateral medulla, a sympathoregulatory brainstem center hyperactive in Vav3-/- mice. Vagal afferent inputs are also required for the development of additional pathophysiological conditions associated with deregulated rostral ventrolateral medulla activity. By contrast, they are dispensable for other peripheral sympathoexcitation-associated disorders sparing metabolic alterations in liver.
Keywords: GABAergic signals; adipose tissue; brainstem; diabetes; hypertension; liver; metabolic syndrome; sympathetic system; thermogenesis; ventrolateral medulla.
Conflict of interest statement
The authors report no conflict of interest associated with this work.
Figures
References
-
- Arner P, Gunnarsson R, Blomdahl S, Groth CG. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care. 1983;6:23–25. - PubMed
-
- Benthem L, Keizer K, Wiegman CH, de Boer SF, Strubbe JH, Steffens AB, Kuipers F, Scheurink AJ. Excess portal venous long-chain fatty acids induce syndrome X via HPA axis and sympathetic activation. Am J Physiol Endocrinol Metab. 2000;279:E1286–1293. - PubMed
-
- Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, Coleman T, Mecham RP, Kelly DP, Semenkovich CF. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003;9:1069–1075. - PubMed
-
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

