Bivalent oral cholera vaccination induces a memory B cell response to the V. cholerae O1-polysaccharide antigen in Haitian adults
- PMID: 30703094
- PMCID: PMC6372202
- DOI: 10.1371/journal.pntd.0007057
Bivalent oral cholera vaccination induces a memory B cell response to the V. cholerae O1-polysaccharide antigen in Haitian adults
Abstract
The bivalent killed whole-cell oral cholera vaccine (BivWC) is being increasingly used to prevent cholera. The presence of O-antigen-specific memory B cells (MBC) has been associated with protective immunity against cholera, yet MBC responses have not been evaluated after BivWC vaccination. To address this knowledge gap, we measured V. cholerae O1-antigen MBC responses following BivWC vaccination. Adults in St. Marc, Haiti, received 2 doses of the BivWC vaccine, Shanchol, two weeks apart. Participants were invited to return at days 7, 21, 44, 90, 180 and 360 after the initial vaccination. Serum antibody and MBC responses were assessed at each time-point before and following vaccination. We observed that vaccination with BivWC resulted in significant O-antigen specific MBC responses to both Ogawa and Inaba serotypes that were detected by day 21 and remained significantly elevated over baseline for up to 12 months following vaccination. The BivWC oral cholera vaccine induces durable MBC responses to the V. cholerae O1-antigen. This suggests that long-term protection observed following vaccination with BivWC could be mediated or maintained by MBC responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

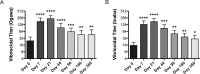



References
-
- Global Task Force on Cholera Control, World Health Organization (WHO). Declaration to Ending Cholera. 2017;: 1–2.
-
- World Health Organization. Deployments from the oral cholera vaccine stockpile, 2013–2017. Wkly Epidemiol Rec. 2017;92: 437–442. - PubMed
-
- Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, et al. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol. 2012;19: 842–848. 10.1128/CVI.00037-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

