Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index
- PMID: 30703352
- PMCID: PMC6499670
- DOI: 10.1016/j.contraception.2019.01.003
Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index
Abstract
Objective: To assess the pharmacokinetics (PK) of levonorgestrel after 1.5 mg oral doses (LNG-EC) in women with normal, obese and extremely obese body mass index (BMI).
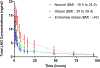
Study design: The 1.5 mg LNG dose was given to healthy, reproductive-age, ovulatory women with normal BMI (mean 22.0), obese (mean 34.4), and extremely obese (mean 46.6 kg/m2) BMI. Total serum LNG was measured over 0 to 96 h by radioimmunoassay while free and bioavailable LNG were calculated. The maximum concentration (Cmax), time to maximum concentration (Tmax), and area under the curve (AUC) of LNG were assessed. Pharmacokinetic parameters calculated included half-life (t1/2), clearance (CL) and volume of distribution (Vss).
Results: Ten normal-BMI, 11 obese-BMI, 5 extremely obese-BMI women were studied. After LNG-EC, mean total LNG metrics were lower in the obese and extremely obese groups compared to normal (Cmax 10.5 and 10.5 versus 16.2 ng/mL, both p<.01; AUC 208 and 197 versus 360 h × ng/mL, both p<.05). Mean bioavailable LNG Cmax was lower in obese (7.03 ng/mL, p<.05) and extremely obese (7.53 ng/ml, p=.198) compared to normal BMI (9.39 ng/mL). Mean bioavailable LNG AUC values were lower in obese and extremely obese compared to normal (131.6 and 127.5 vs 185.0 h × ng/mL, p<.05 for both).
Conclusions: Obese and extremely obese women were exposed to lower total and bioavailable LNG than normal BMI women.
Implications: Lower 'bioavailable' (free plus albumin bound) LNG AUC in obese women may play a role in the purported reduced efficacy of LNG-EC in obese users.
Keywords: BAI; BMI; Emergency contraception; Levonorgestrel; Obesity; Pharmacokinetics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


References
-
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–7. - PubMed
-
- Kapp N, Abitbol JL, Mathe H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91(2):97–104. - PubMed
-
- Gemzell-Danielsson K, Kardos L, von Hertzen H. Impact of bodyweight/body mass index on the effectiveness of emergency contraception with levonorgestrel: a pooled-analysis of three randomized controlled trials. Curr Med Res Opin. 2015;31(12):2241–8. - PubMed
-
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95(5):464–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

