Variability in the management of line-related upper extremity deep vein thrombosis
- PMID: 30704347
- PMCID: PMC7012441
- DOI: 10.1177/0268355519827155
Variability in the management of line-related upper extremity deep vein thrombosis
Abstract
Objectives:: Central-venous devices are risk-factors for upper extremity deep vein thrombosis. We surveyed physicians to identify practice-patterns and adherence to American College of Chest Physicians guidelines.
Methods:: The 13-question survey obtained physician-demographics and treatment-choices. Respondents were grouped into surgical and medical specialists. Data were reported as ratios and percentages, and compared using Fisher’s exact test.
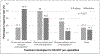
Results:: We received 143 responses from physicians; 65% treated one-to-two new cases/month. Most physicians (69.2%) used anticoagulation; 36.4% retained the catheter and 32.9% removed it. Medical-specialists retained catheters more often than surgeons (p = 0.027). For recurrences, 84% repeated anticoagulation; 50.3% retained the catheter. A majority anticoagulated upper-extremity deep-vein thrombosis in long-term catheters for three months only (55.1%). Direct oral anticoagulants were used frequently (43.6%). Only 10% believed that existing guidelines were appropriate and only 2.8% followed all guidelines.
Conclusion:: There is great variability in treatment-decisions for upper-extremity deep-vein thrombosis. The existing guidelines are considered inadequate and not followed by most physicians.
Keywords: Deep vein thrombosis; anticoagulation; catheter; intravenous device; upper extremity.
Conflict of interest statement
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


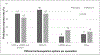
References
-
- Lamontagne F, McIntyre L, Dodek P, et al. Nonleg venous thrombosis in critically ill adults. JAMA Intern Med 2014; 174: 689. - PubMed
-
- Young AM, Billingham LJ, Begum G, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet 2009; 373: 567–574. - PubMed
-
- Lavau-Denes S, Lacroix P, Maubon A, et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol 2013; 72: 65–73. - PubMed
-
- Kovacs MJ, Kahn SR, Rodger M, et al. A pilot study of central venous catheter survival in cancer patients using low-molecular-weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study). J Thromb Haemost 2007; 5: 1650–1653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

