Study protocol: optimized complementary feeding study (OTIS): a randomized controlled trial of the impact of a protein-reduced complementary diet based on Nordic foods
- PMID: 30704429
- PMCID: PMC6357470
- DOI: 10.1186/s12889-019-6466-1
Study protocol: optimized complementary feeding study (OTIS): a randomized controlled trial of the impact of a protein-reduced complementary diet based on Nordic foods
Abstract
Background: What we eat as infants and children carries long-term consequences. Apart from breastfeeding, the composition of the complementary diet, i.e. the foods given to the infant during the transition from breast milk/infant formula to regular family foods affects the child's future health. A high intake of protein, a low intake of fruits, vegetables and fish and an unfavorable distribution between polyunsaturated and saturated fats are considered to be associate with health risks, e.g. obesity, type 2 diabetes and dyslipidemia later in life.
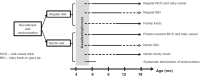
Methods: In a randomized, controlled study from 6 to 18 months of age we will compare the currently recommended, Swedish complementary diet to one based on Nordic foods, i.e. an increased intake of fruits, berries, vegetables, tubers, whole-grain and game, and a lower intake of sweets, dairy, meat and poultry, with lower protein content (30% decrease), a higher intake of vegetable fats and fish and a systematic introduction of fruits and greens. The main outcomes are body composition (fat and fat-free mass measured with deuterium), metabolic and inflammatory biomarkers (associated with the amount of body fat) in blood and urine, gut microbiota (thought to be the link between early diet, metabolism and diseases such as obesity and insulin resistance) and blood pressure. We will also measure the participants' energy and nutrient intake, eating behavior and temperament through validated questionnaires, acceptance of new and unfamiliar foods through video-taped test meals and assessment of cognitive development, which we believe can be influenced through an increased intake of fish and milk fats, notably milk fat globule membranes (MFGM).
Discussion: If the results are what we expect, i.e. improved body composition and a less obesogenic, diabetogenic and inflammatory metabolism and gut microbiota composition, a more sustainable nutrient intake for future health and an increased acceptance of healthy foods, they will have a profound impact on the dietary recommendations to infants in Sweden and elsewhere, their eating habits later in life and subsequently their long-term health.
Trial registration: NCT02634749 . Registration date 18 December 2015.
Keywords: Body composition; Child development; Child nutrition physiology; Feeding behavior; Food preference; Growth; Hypertension; Infant food; Insulin resistance; Microbiota; Obesity.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval was granted from the Regional Ethical Review Board at Umeå University, Sweden (Dnr 2014–363-31 M, 2016–134-32 M and 2018-4-32). Informed consent has been obtained from the parents/legal guardians of all participants.
Consent for publication
Not applicable.
Competing interests
UJ is a doctoral student at the Umeå University Industrial Doctoral School for Research and Innovation with Semper AB as industrial sponsor. CT is an employee of Semper AB. OH and BL are members of the Scientific Advisory Board of Semper AB. TL, OH and BL have received research support from Semper. The remaining authors declare no conflicts of interest related to the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Protein-Reduced Complementary Foods Based on Nordic Ingredients Combined with Systematic Introduction of Taste Portions Increase Intake of Fruits and Vegetables in 9 Month Old Infants: A Randomised Controlled Trial.Nutrients. 2019 Jun 2;11(6):1255. doi: 10.3390/nu11061255. Nutrients. 2019. PMID: 31159495 Free PMC article. Clinical Trial.
-
A randomized, controlled trial of a Nordic, protein-reduced complementary diet in infants: effects on body composition, growth, biomarkers, and dietary intake at 12 and 18 months.Am J Clin Nutr. 2023 Jun;117(6):1219-1231. doi: 10.1016/j.ajcnut.2023.03.020. Epub 2023 Mar 27. Am J Clin Nutr. 2023. PMID: 36990225 Clinical Trial.
-
Lessons from the feeding infants and toddlers study in North America: what children eat, and implications for obesity prevention.Ann Nutr Metab. 2013;62 Suppl 3:27-36. doi: 10.1159/000351538. Epub 2013 Aug 19. Ann Nutr Metab. 2013. PMID: 23970213
-
[Simple obesity in children. A study on the role of nutritional factors].Med Wieku Rozwoj. 2006 Jan-Mar;10(1):3-191. Med Wieku Rozwoj. 2006. PMID: 16733288 Review. Polish.
-
A review of dairy food intake for improving health among black infants, toddlers, and young children in the US.J Natl Med Assoc. 2024 Apr;116(2 Pt 2):228-240. doi: 10.1016/j.jnma.2024.01.014. Epub 2024 Feb 15. J Natl Med Assoc. 2024. PMID: 38360504 Review.
Cited by
-
Towards a More Sustainable Nutrition: Complementary Feeding and Early Taste Experiences as a Basis for Future Food Choices.Nutrients. 2021 Aug 4;13(8):2695. doi: 10.3390/nu13082695. Nutrients. 2021. PMID: 34444855 Free PMC article.
-
Impact of a "vegetables first" approach to complementary feeding on later intake and liking of vegetables in infants: a study protocol for a randomised controlled trial.Trials. 2021 Jul 26;22(1):488. doi: 10.1186/s13063-021-05374-7. Trials. 2021. PMID: 34311749 Free PMC article.
-
Starting complementary feeding with vegetables only increases vegetable acceptance at 9 months: a randomized controlled trial.Am J Clin Nutr. 2022 Jul 6;116(1):111-121. doi: 10.1093/ajcn/nqac080. Am J Clin Nutr. 2022. PMID: 35679432 Free PMC article. Clinical Trial.
-
The Immunological Role of Milk Fat Globule Membrane.Nutrients. 2022 Oct 31;14(21):4574. doi: 10.3390/nu14214574. Nutrients. 2022. PMID: 36364836 Free PMC article. Review.
-
The Role of Gut Microbiota in Pediatric Obesity and Metabolic Disorders: Insights from a Comprehensive Review.Nutrients. 2025 May 30;17(11):1883. doi: 10.3390/nu17111883. Nutrients. 2025. PMID: 40507152 Free PMC article. Review.
References
-
- Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, hepatology, and nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119–132. doi: 10.1097/MPG.0000000000001454. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical