Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR)
- PMID: 30704493
- PMCID: PMC6357474
- DOI: 10.1186/s13058-018-1091-y
Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR)
Abstract
Background: Patients with early breast cancer (EBC) achieving pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT) have a favorable prognosis. Breast surgery might be avoided in patients in whom the presence of residual tumor can be ruled out with high confidence. Here, we investigated the diagnostic accuracy of contrast-enhanced MRI (CE-MRI) in predicting pCR and long-term outcome after NACT.
Methods: Patients with EBC, including patients with locally advanced disease, who had undergone CE-MRI after NACT, were retrospectively analyzed (n = 246). Three radiologists, blinded to clinicopathologic data, reevaluated all MRI scans regarding to the absence (radiologic complete remission; rCR) or presence (no-rCR) of residual contrast enhancement. Clinical and pathologic responses were compared categorically using Cohen's kappa statistic. The Kaplan-Meier method was used to estimate recurrence-free survival (RFS) and overall survival (OS).
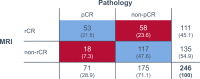
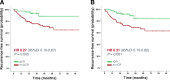
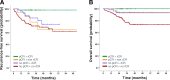
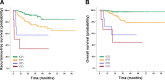
Results: Overall rCR and pCR (no invasive tumor in the breast and axilla (ypT0/is N0)) rates were 45% (111/246) and 29% (71/246), respectively. Only 48% (53/111; 95% CI 38-57%) of rCR corresponded to a pCR (= positive predictive value - PPV). Conversely, in 87% (117/135; 95% CI 79-92%) of patients, residual tumor observed on MRI was pathologically confirmed (= negative predictive value - NPV). Sensitivity to detect a pCR was 75% (53/71; 95% CI 63-84%), while specificity to detect residual tumor and accuracy were 67% (117/175; 95% CI 59-74%) and 69% (170/246; 95% CI 63-75%), respectively. The PPV was significantly lower in hormone-receptor (HR)-positive compared to HR-negative tumors (17/52 = 33% vs. 36/59 = 61%; P = 0.004). The concordance between rCR and pCR was low (Cohen's kappa - 0.1), however in multivariate analysis both assessments were significantly associated with RFS (rCR P = 0.037; pCR P = 0.033) and OS (rCR P = 0.033; pCR P = 0.043).
Conclusion: Preoperative CE-MRI did not accurately predict pCR after NACT for EBC, especially not in HR-positive tumors. However, rCR was strongly associated with favorable RFS and OS.
Keywords: Breast cancer; MRI; Neoadjuvant chemotherapy; Prediction of complete pathologic response; Survival.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the province Salzburg, which waived the requirement to obtain informed consent (IRB number: 415-EP/73/648–2016).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. - DOI - PubMed
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Curigliano G, Burstein HJ, E PW, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–1712. doi: 10.1093/annonc/mdx308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

