Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways
- PMID: 30704535
- PMCID: PMC6357421
- DOI: 10.1186/s13287-019-1152-x
Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways
Abstract
Background: Human adipose stem cells (ASCs) have emerged as a promising treatment paradigm for skin wounds. Recent works demonstrate that the therapeutic effect of stem cells is partially mediated by extracellular vesicles, which comprise exosomes and microvesicles. In this study, we investigate the regenerative effects of isolated microvesicles from ASCs and evaluate the mechanisms how ASC microvesicles promote wound healing.
Methods: Adipose stem cell-derived microvesicles (ASC-MVs) were isolated by differential ultracentrifugation, stained by PKH26, and characterized by electron microscopy and dynamic light scattering (DLS). We examined ASC-MV effects on proliferation, migration, and angiogenesis of keratinocytes, fibroblasts, and endothelial cells both in vitro and in vivo. Next, we explored the underlying mechanisms by gene expression analysis and the activation levels of AKT and ERK signaling pathways in all three kinds of cells after ASC-MV stimulation. We then assessed the effect of ASC-MVs on collagen deposition, neovascularization, and re-epithelialization in an in vivo skin injury model.
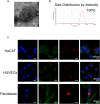
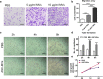
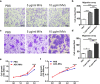
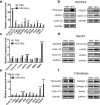
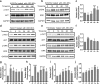
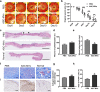
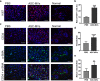
Results: ASC-MVs could be readily internalized by human umbilical vein endothelial cells (HUVECs), HaCAT, and fibroblasts and significantly promoted the proliferation, migration, and angiogenesis of these cells both in vitro and in vivo. The gene expression of proliferative markers (cyclin D1, cyclin D2, cyclin A1, cyclin A2) and growth factors (VEGFA, PDGFA, EGF, FGF2) was significantly upregulated after ASC-MV treatment. Importantly, ASC-MVs stimulated the activation of AKT and ERK signaling pathways in those cells. The local injection of ASC-MVs at wound sites significantly increased the re-epithelialization, collagen deposition, and neovascularization and led to accelerated wound closure.
Conclusions: Our data suggest that ASC-MVs can stimulate HUVEC, HaCAT, and fibroblast functions. ASC-MV therapy significantly accelerates wound healing, and the benefits of ASC-MVs may due to the involvement of AKT and ERK signaling pathways. This illustrates the therapeutic potential of ASC-MVs which may become a novel treatment paradigm for cutaneous wound healing.
Keywords: Adipose stem cells (ASCs); Extracellular vesicles; Microvesicles (MVs); Wound healing.
Conflict of interest statement
Ethics approval and consent to participate
All animal experiments were conducted according to the guidelines and standards of the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology.
The use of human adipose tissue and foreskin was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No. 2018-S288). We obtained the written informed consent from all the patients participated in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

