Applicability of in vivo staging of regional amyloid burden in a cognitively normal cohort with subjective memory complaints: the INSIGHT-preAD study
- PMID: 30704537
- PMCID: PMC6357385
- DOI: 10.1186/s13195-019-0466-3
Applicability of in vivo staging of regional amyloid burden in a cognitively normal cohort with subjective memory complaints: the INSIGHT-preAD study
Erratum in
-
Correction: Applicability of in vivo staging of regional amyloid burden in a cognitively normal cohort with subjective memory complaints: the INSIGHT-preAD study.Alzheimers Res Ther. 2022 Sep 14;14(1):131. doi: 10.1186/s13195-022-01025-4. Alzheimers Res Ther. 2022. PMID: 36104713 Free PMC article. No abstract available.
Abstract
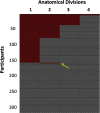
Background: Current methods of amyloid PET interpretation based on the binary classification of global amyloid signal fail to identify early phases of amyloid deposition. A recent analysis of 18F-florbetapir PET data from the Alzheimer's disease Neuroimaging Initiative cohort suggested a hierarchical four-stage model of regional amyloid deposition that resembles neuropathologic estimates and can be used to stage an individual's amyloid burden in vivo. Here, we evaluated the validity of this in vivo amyloid staging model in an independent cohort of older people with subjective memory complaints (SMC). We further examined its potential association with subtle cognitive impairments in this population at elevated risk for Alzheimer's disease (AD).
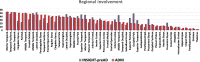
Methods: The monocentric INSIGHT-preAD cohort includes 318 cognitively intact older individuals with SMC. All individuals underwent 18F-florbetapir PET scanning and extensive neuropsychological testing. We projected the regional amyloid uptake signal into the previously proposed hierarchical staging model of in vivo amyloid progression. We determined the adherence to this model across all cases and tested the association between increasing in vivo amyloid stage and cognitive performance using ANCOVA models.
Results: In total, 156 participants (49%) showed evidence of regional amyloid deposition, and all but 2 of these (99%) adhered to the hierarchical regional pattern implied by the in vivo amyloid progression model. According to a conventional binary classification based on global signal (SUVRCereb = 1.10), individuals in stages III and IV were classified as amyloid-positive (except one in stage III), but 99% of individuals in stage I and even 28% of individuals in stage II were classified as amyloid-negative. Neither in vivo amyloid stage nor conventional binary amyloid status was significantly associated with cognitive performance in this preclinical cohort.
Conclusions: The proposed hierarchical staging scheme of PET-evidenced amyloid deposition generalizes well to data from an independent cohort of older people at elevated risk for AD. Future studies will determine the prognostic value of the staging approach for predicting longitudinal cognitive decline in older individuals at increased risk for AD.
Keywords: Amyloid PET; In vivo staging; Subjective memory complaint.
Conflict of interest statement
Ethics approval and consent to participate
The ethics committee of the Pitié-Salpêtrière University Hospital approved the study protocol. All participants signed an informed consent form, given and explained to them 2 weeks before enrolment.
Competing interests
The authors declare that they have no competing interests. HH serves as the Senior Associate Editor for the Journal Alzheimer’s and Dementia; he received lecture fees from Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics and Roche Diagnostics.
HH is a co-inventor in the following patents as a scientific expert and has received no royalties:
• In Vitro Multi-parameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388.
• In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784.
• Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300.
• In Vitro Multi-parameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463.
• In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286.
• In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822.
• In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553.
• CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797.
• In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966.
• Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures