A Photoactivatable Botulinum Neurotoxin for Inducible Control of Neurotransmission
- PMID: 30704911
- PMCID: PMC6524650
- DOI: 10.1016/j.neuron.2019.01.002
A Photoactivatable Botulinum Neurotoxin for Inducible Control of Neurotransmission
Abstract
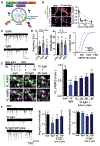
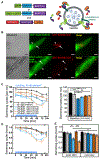
Regulated secretion is critical for diverse biological processes ranging from immune and endocrine signaling to synaptic transmission. Botulinum and tetanus neurotoxins, which specifically proteolyze vesicle fusion proteins involved in regulated secretion, have been widely used as experimental tools to block these processes. Genetic expression of these toxins in the nervous system has been a powerful approach for disrupting neurotransmitter release within defined circuitry, but their current utility in the brain and elsewhere remains limited by lack of spatial and temporal control. Here we engineered botulinum neurotoxin B so that it can be activated with blue light. We demonstrate the utility of this approach for inducibly disrupting excitatory neurotransmission, providing a first-in-class optogenetic tool for persistent, light-triggered synaptic inhibition. In addition to blocking neurotransmitter release, this approach will have broad utility for conditionally disrupting regulated secretion of diverse bioactive molecules, including neuropeptides, neuromodulators, hormones, and immune molecules. VIDEO ABSTRACT.
Keywords: Optogenetics; SNARE protein; botulinum toxin; iLID; neurotransmitter; secretion; synapse; tetanus toxin; vamp2; vesicle.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
A provisional patent application has been filed related to this study.
Figures




References
-
- Abraham C, Hutter H, Palfreyman MT, Spatkowski G, Weimer RM, Windoffer R, Jorgensen EM, and Leube RE (2006). Synaptic tetraspan vesicle membrane proteins are conserved but not needed for synaptogenesis and neuronal function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 103, 8227–8232. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

