Endonuclease and redox activities of human apurinic/apyrimidinic endonuclease 1 have distinctive and essential functions in IgA class switch recombination
- PMID: 30705092
- PMCID: PMC6442068
- DOI: 10.1074/jbc.RA118.006601
Endonuclease and redox activities of human apurinic/apyrimidinic endonuclease 1 have distinctive and essential functions in IgA class switch recombination
Abstract
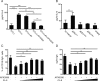
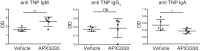
The base excision repair (BER) pathway is an important DNA repair pathway and is essential for immune responses. In fact, it regulates both the antigen-stimulated somatic hypermutation (SHM) process and plays a central function in the process of class switch recombination (CSR). For both processes, a central role for apurinic/apyrimidinic endonuclease 1 (APE1) has been demonstrated. APE1 acts also as a master regulator of gene expression through its redox activity. APE1's redox activity stimulates the DNA-binding activity of several transcription factors, including NF-κB and a few others involved in inflammation and in immune responses. Therefore, it is possible that APE1 has a role in regulating the CSR through its function as a redox coactivator. The present study was undertaken to address this question. Using the CSR-competent mouse B-cell line CH12F3 and a combination of specific inhibitors of APE1's redox (APX3330) and repair (compound 3) activities, APE1-deficient or -reconstituted cell lines expressing redox-deficient or endonuclease-deficient proteins, and APX3330-treated mice, we determined the contributions of both endonuclease and redox functions of APE1 in CSR. We found that APE1's endonuclease activity is essential for IgA-class switch recombination. We provide evidence that the redox function of APE1 appears to play a role in regulating CSR through the interleukin-6 signaling pathway and in proper IgA expression. Our results shed light on APE1's redox function in the control of cancer growth through modulation of the IgA CSR process.
Keywords: DNA endonuclease; DNA transcription; apurinic/apyrimidinic endonuclease 1 (APE1); base excision repair (BER); class switch recombination; immunoglobulin A (IgA); immunology; redox-inhibitor.
© 2019 Frossi et al.
Conflict of interest statement
Mark R. Kelley is Chief Scientific Officer of Apexian Pharmaceuticals, the biotech company that has licensed APX3330 used in these studies. Apexian Pharmaceuticals had neither control nor oversight of the studies, interpretation, or presentation of the data in this manuscript. They did not have to approve the manuscript in any way prior to its submission
Figures


Similar articles
-
Apurinic/apyrimidinic endonuclease 1 (APE1) is dispensable for activation-induced cytidine deaminase (AID)-dependent somatic hypermutation in the immunoglobulin gene.Int Immunol. 2019 Jul 30;31(8):543-554. doi: 10.1093/intimm/dxz028. Int Immunol. 2019. PMID: 30877298
-
APE1 is dispensable for S-region cleavage but required for its repair in class switch recombination.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17242-7. doi: 10.1073/pnas.1420221111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404348 Free PMC article.
-
Apurinic/apyrimidinic endonuclease 1 is the essential nuclease during immunoglobulin class switch recombination.Mol Cell Biol. 2013 Apr;33(7):1468-73. doi: 10.1128/MCB.00026-13. Epub 2013 Feb 4. Mol Cell Biol. 2013. PMID: 23382073 Free PMC article.
-
Chemically induced partial unfolding of the multifunctional apurinic/apyrimidinic endonuclease 1.Protein Sci. 2025 Jun;34(6):e70148. doi: 10.1002/pro.70148. Protein Sci. 2025. PMID: 40371780 Free PMC article. Review.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
Cited by
-
APE1 and NPM1 protect cancer cells from platinum compounds cytotoxicity and their expression pattern has a prognostic value in TNBC.J Exp Clin Cancer Res. 2019 Jul 15;38(1):309. doi: 10.1186/s13046-019-1294-9. J Exp Clin Cancer Res. 2019. PMID: 31307523 Free PMC article.
-
Molecular Mechanisms Regulating the DNA Repair Protein APE1: A Focus on Its Flexible N-Terminal Tail Domain.Int J Mol Sci. 2021 Jun 11;22(12):6308. doi: 10.3390/ijms22126308. Int J Mol Sci. 2021. PMID: 34208390 Free PMC article. Review.
-
Endogenous oxidized DNA bases and APE1 regulate the formation of G-quadruplex structures in the genome.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11409-11420. doi: 10.1073/pnas.1912355117. Epub 2020 May 13. Proc Natl Acad Sci U S A. 2020. PMID: 32404420 Free PMC article.
-
Functional Role of N-Terminal Extension of Human AP Endonuclease 1 In Coordination of Base Excision DNA Repair via Protein-Protein Interactions.Int J Mol Sci. 2020 Apr 28;21(9):3122. doi: 10.3390/ijms21093122. Int J Mol Sci. 2020. PMID: 32354179 Free PMC article.
-
Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer.Cell Death Dis. 2021 May 18;12(6):503. doi: 10.1038/s41419-021-03804-7. Cell Death Dis. 2021. PMID: 34006852 Free PMC article.
References
-
- Vuong B. Q., Herrick-Reynolds K., Vaidyanathan B., Pucella J. N., Ucher A. J., Donghia N. M., Gu X., Nicolas L., Nowak U., Rahman N., Strout M. P., Mills K. D., Stavnezer J., and Chaudhuri J. (2013) A DNA break- and phosphorylation-dependent positive feedback loop promotes immunoglobulin class-switch recombination. Nat. Immunol. 14, 1183–1189 10.1038/ni.2732 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

