Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses
- PMID: 30705117
- PMCID: PMC6446704
- DOI: 10.1194/jlr.S091793
Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses
Abstract
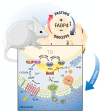
Although counterregulatory hormones and mediators of the fight-or-flight responses are well defined at many levels, how energy stores per se are integrated into this system remains an enigmatic question. Recent years have seen the adipose tissue become a central focus for mediating intracellular signaling and communication through the release of a variety of bioactive lipids and substrates, as well as various adipokines. A critical integration node among these mediators and responses is controlled by FA binding protein 4 (FABP4), also known as adipocyte protein 2 (aP2), which is highly expressed in adipose tissue and functions as a lipid chaperone protein. Recently, it was demonstrated that FABP4 is a secreted hormone that has roles in maintaining glucose homeostasis, representing a key juncture facilitating communication between energy-storage systems and distant organs to respond to life-threatening situations. However, chronic engagement of FABP4 under conditions of immunometabolic stress, such as obesity, exacerbates a number of immunometabolic diseases, including diabetes, asthma, cancer, and atherosclerosis. In both preclinical mouse models and humans, levels of circulating FABP4 have been correlated with metabolic disease incidence, and reducing FABP4 levels or activity is associated with improved metabolic health. In this review, we will discuss the intriguing emerging biology of this protein, including potential therapeutic options for targeting circulating FABP4.
Keywords: immunometabolism; metabolism; obesity.
Copyright © 2019 Prentice et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
G.S.H. is on the Scientific Advisory Boards of Seven Bridges Corporation and Crescenta Pharmaceuticals and holds equity in the latter. The G.S.H. lab receives sponsored research funds from Servier for research unrelated to the content of this manuscript. The other authors have no conflicts of interest to declare.
Figures

References
-
- Matarese V., and Bernlohr D. A.. 1988. Purification of murine adipocyte lipid-binding protein. Characterization as a fatty acid- and retinoic acid-binding protein. J. Biol. Chem. 263: 14544–14551. - PubMed
-
- Jenkins-Kruchten A. E., Bennaars-Eiden A., Ross J. R., Shen W. J., Kraemer F. B., and Bernlohr D. A.. 2003. Fatty acid-binding protein-hormone-sensitive lipase interaction. Fatty acid dependence on binding. J. Biol. Chem. 278: 47636–47643. - PubMed
-
- Schroeder F., Petrescu A. D., Huang H., Atshaves B. P., McIntosh A. L., Martin G. G., Hostetler H. A., Vespa A., Landrock D., Landrock K. K., et al. 2008. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 43: 1–17. - PubMed
-
- Hofer P., Boeszoermenyi A., Jaeger D., Feiler U., Arthanari H., Mayer N., Zehender F., Rechberger G., Oberer M., Zimmermann R., et al. 2015. Fatty acid-binding proteins interact with comparative gene identification-58 linking lipolysis with lipid ligand shuttling. J. Biol. Chem. 290: 18438–18453. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

