Opposing reactions in coenzyme A metabolism sensitize Mycobacterium tuberculosis to enzyme inhibition
- PMID: 30705156
- PMCID: PMC6613350
- DOI: 10.1126/science.aau8959
Opposing reactions in coenzyme A metabolism sensitize Mycobacterium tuberculosis to enzyme inhibition
Erratum in
-
Erratum for the Research Article "Opposing reactions in coenzyme A metabolism sensitize Mycobacterium tuberculosis to enzyme inhibition" by E. Ballinger, J. Mosior, T. Hartman, K. Burns-Huang, B. Gold, R. Morris, L. Goullieux, I. Blanc, J. Vaubourgeix, S. Lagrange, L. Fraisse, S. Sans, C. Couturier, E. Bacqué, K. Rhee, S. M. Scarry, J. Aubé, G. Yang, O. Ouerfelli, D. Schnappinger, T. R. Ioerger, C. A. Engelhart, J. A. McConnell, K. McAulay, A. Fay, C. Roubert, J. Sacchettini, C. Nathan.Science. 2019 Jun 21;364(6446):eaay3546. doi: 10.1126/science.aay3546. Science. 2019. PMID: 31221826 No abstract available.
Abstract
Mycobacterium tuberculosis (Mtb) is the leading infectious cause of death in humans. Synthesis of lipids critical for Mtb's cell wall and virulence depends on phosphopantetheinyl transferase (PptT), an enzyme that transfers 4'-phosphopantetheine (Ppt) from coenzyme A (CoA) to diverse acyl carrier proteins. We identified a compound that kills Mtb by binding and partially inhibiting PptT. Killing of Mtb by the compound is potentiated by another enzyme encoded in the same operon, Ppt hydrolase (PptH), that undoes the PptT reaction. Thus, loss-of-function mutants of PptH displayed antimicrobial resistance. Our PptT-inhibitor cocrystal structure may aid further development of antimycobacterial agents against this long-sought target. The opposing reactions of PptT and PptH uncover a regulatory pathway in CoA physiology.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
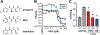
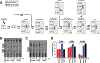



Comment in
-
Expanding the anti-TB arsenal.Science. 2019 Feb 1;363(6426):457-458. doi: 10.1126/science.aaw5224. Science. 2019. PMID: 30705174 No abstract available.
References
-
- Walsh C, Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000). - PubMed
-
- Moolman WJ, de Villiers M, Strauss E, Recent advances in targeting coenzyme A biosynthesis and utilization for antimicrobial drug development. Biochem Soc Trans 42, 1080–1086 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

