Kaposi sarcoma
- PMID: 30705286
- PMCID: PMC6685213
- DOI: 10.1038/s41572-019-0060-9
Kaposi sarcoma
Abstract
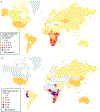
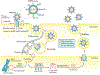
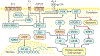
Kaposi sarcoma (KS) gained public attention as an AIDS-defining malignancy; its appearance on the skin was a highly stigmatizing sign of HIV infection during the height of the AIDS epidemic. The widespread introduction of effective antiretrovirals to control HIV by restoring immunocompetence reduced the prevalence of AIDS-related KS, although KS does occur in individuals with well-controlled HIV infection. KS also presents in individuals without HIV infection in older men (classic KS), in sub-Saharan Africa (endemic KS) and in transplant recipients (iatrogenic KS). The aetiologic agent of KS is KS herpesvirus (KSHV; also known as human herpesvirus-8), and viral proteins can induce KS-associated cellular changes that enable the virus to evade the host immune system and allow the infected cell to survive and proliferate despite viral infection. Currently, most cases of KS occur in sub-Saharan Africa, where KSHV infection is prevalent owing to transmission by saliva in childhood compounded by the ongoing AIDS epidemic. Treatment for early AIDS-related KS in previously untreated patients should start with the control of HIV with antiretrovirals, which frequently results in KS regression. In advanced-stage KS, chemotherapy with pegylated liposomal doxorubicin or paclitaxel is the most common treatment, although it is seldom curative. In sub-Saharan Africa, KS continues to have a poor prognosis. Newer treatments for KS based on the mechanisms of its pathogenesis are being explored.
Figures
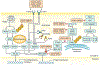
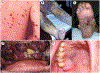
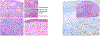
References
-
- Kaposi M Idiopatisches multiples pigmentsarkom der haut [German]. Arch. Dermatol. Syph. 4, 265–273 (1872).
-
- Montpellier J & Mussini-Montpellier J Le Cancer en France d’outre-mer: Considérations Pathogéniques (Libraire Ferraris, 1947).
-
- D’Oliveira JJ & Torres FO Kaposi’s sarcoma in the Bantu of Mozambique. Cancer 30, 553–561 (1972). - PubMed
-
- Thijs A L’angiosarcomatose de Kaposi au Congo Beige et au Ruanda-Urundi [French]. Ann. Soc. Belg. Med. Trop 37, 295–307 (1957). - PubMed
-
-
Gottlieb GJ et al. A preliminary communication on extensively disseminated Kaposi’s sarcoma in young homosexual men. Am. J. Dermatopathol. 3, 111–114 (1981).
This paper presents the first report of KS in MSM as a harbinger of the AIDS epidemic.
-

