Transient increase of activated regulatory T cells early after kidney transplantation
- PMID: 30705299
- PMCID: PMC6355855
- DOI: 10.1038/s41598-018-37218-x
Transient increase of activated regulatory T cells early after kidney transplantation
Abstract
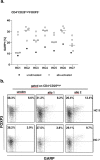
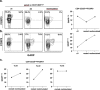
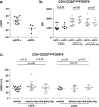
Regulatory T cells (Tregs) are crucial in controlling allospecific immune responses. However, studies in human kidney recipients regarding the contribution of polyspecific Tregs have provided differing results and studies on alloreactive Tregs are missing completely. In this retrospective study, we specifically analyzed activated CD4+CD25highFOXP3+GARP+ Tregs in 17 patients of a living donor kidney transplantation cohort longitudinally over 24 months by flow cytometry (FOXP3: forkhead box protein 3, GARP: glycoprotein A repetitions predominant). We could demonstrate that Tregs of patients with end-stage renal disease (ESRD) are already pre-activated when compared to healthy controls. Furthermore, even though total CD4+CD25highFOXP3+ Treg numbers decreased in the first three months after transplantation, frequency of activated Tregs increased significantly representing up to 40% of all peripheral Tregs. In a cohort of living donor kidney transplantation recipients with stable graft function, frequencies of activated Tregs did not correlate with the occurrence of acute cellular rejection or chronic graft dysfunction. Our results will be important for clinical trials using adoptive Treg therapy after kidney transplantation. Adoptively transferred Tregs could be important to compensate the Treg loss at month 3, while they have to compete within the Treg niche with a large number of activated Tregs.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Jaeckel, E., von Boehmer, H. & Manns, M. P. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes54, 306-310, 54.02.04.db04-1252 (2005). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

