Memory-like Liver Natural Killer Cells are Responsible for Islet Destruction in Secondary Islet Transplantation
- PMID: 30705364
- PMCID: PMC6355863
- DOI: 10.1038/s41598-018-37395-9
Memory-like Liver Natural Killer Cells are Responsible for Islet Destruction in Secondary Islet Transplantation
Abstract
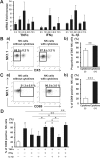
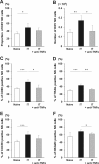
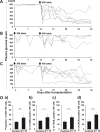
We previously demonstrated the pivotal role of natural killer (NK) cells in islet graft loss during the early phase after intraportal syngeneic islet transplantation (IT). Liver-resident DX5- NK cells were reported to possess memory-like properties, distinguishing them from conventional DX5+ NK cells. Here, we investigated the impact of primary IT-induced liver DX5- NK cells on the engraftment of secondary-transplanted islets in mice. The culture of liver NK cells isolated from naive mice with TNF-α, IFN-γ, and IL-lβ, mimicking instant blood-mediated inflammatory reaction, led to significantly increased DX5- NK cell percentage among total liver NK cells. Consistently, the prolonged expansion of DX5- CD69+ TRAIL+ CXCR3+ NK cells was observed after intraportal IT of 300 syngeneic islets (marginal mass). In most diabetic mice, 400 syngeneic islets of primary IT were sufficient to achieve normoglycaemia, whereas the same mass after secondary IT failed to induce normoglycaemia in mice that received 200 syngeneic islets during primary IT. These findings indicated that liver-resident DX5- NK cells significantly expanded even after syngeneic IT, and that these memory-like NK cells may target both originally engrafted and secondary-transplanted islets. Furthermore, anti-TNF-α treatment suppressed the expansion of liver-resident DX5- NK cells, resulting in successful islet engraftment after sequential ITs.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Role of Natural Killer Cells in the Innate Immune System After Intraportal Islet Transplantation in Mice.Transplant Proc. 2017 Jan-Feb;49(1):139-144. doi: 10.1016/j.transproceed.2016.10.010. Transplant Proc. 2017. PMID: 28104122
-
Liver natural killer cells play a role in the destruction of islets after intraportal transplantation.Transplantation. 2011 May 15;91(9):952-60. doi: 10.1097/TP.0b013e3182139dc1. Transplantation. 2011. PMID: 21389902
-
Enhancement of islet engraftment and achievement of long-term islet allograft survival by Toll-like receptor 4 blockade.Transplantation. 2015 Jan;99(1):29-35. doi: 10.1097/TP.0000000000000468. Transplantation. 2015. PMID: 25340601
-
Antibody-induced NKG2D blockade in a rat model of intraportal islet transplantation leads to a deleterious reaction.Transpl Int. 2020 Jun;33(6):675-688. doi: 10.1111/tri.13589. Epub 2020 Feb 21. Transpl Int. 2020. PMID: 32003082
-
Memory NK cells: why do they reside in the liver?Cell Mol Immunol. 2013 May;10(3):196-201. doi: 10.1038/cmi.2013.8. Epub 2013 Apr 8. Cell Mol Immunol. 2013. PMID: 23563088 Free PMC article. Review.
Cited by
-
No Time to Die-How Islets Meet Their Demise in Transplantation.Cells. 2023 Mar 3;12(5):796. doi: 10.3390/cells12050796. Cells. 2023. PMID: 36899932 Free PMC article. Review.
-
Single-Cell Landscape of Mouse Islet Allograft and Syngeneic Graft.Front Immunol. 2022 Jun 10;13:853349. doi: 10.3389/fimmu.2022.853349. eCollection 2022. Front Immunol. 2022. PMID: 35757709 Free PMC article.
-
Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation.J Microbiol Biotechnol. 2020 Aug 28;30(8):1132-1141. doi: 10.4014/jmb.2002.02044. J Microbiol Biotechnol. 2020. PMID: 32423189 Free PMC article.
-
Role of liver-resident NK cells in liver immunity.Hepatol Int. 2025 Apr;19(2):315-324. doi: 10.1007/s12072-025-10778-7. Epub 2025 Feb 1. Hepatol Int. 2025. PMID: 39893278 Free PMC article. Review.
-
CD1d-independent NK1.1+ Treg cells are IL2-inducible Foxp3+ T cells co-expressing immunosuppressive and cytotoxic molecules.Front Immunol. 2022 Sep 13;13:951592. doi: 10.3389/fimmu.2022.951592. eCollection 2022. Front Immunol. 2022. PMID: 36177042 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

