PES1 promotes the occurrence and development of papillary thyroid cancer by upregulating the ERα/ERβ protein ratio
- PMID: 30705367
- PMCID: PMC6355968
- DOI: 10.1038/s41598-018-37648-7
PES1 promotes the occurrence and development of papillary thyroid cancer by upregulating the ERα/ERβ protein ratio
Abstract
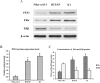
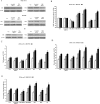
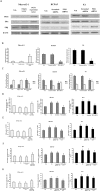
PES1, a BRCT domain-containing protein, has been shown to play a role in modulating the balance and ratio between ERα and ERβ protein, which is involved in the occurrence and development of breast and ovarian cancer. However, its role in connection with the balance and ratio between ERα and ERβ protein in papillary thyroid cancer (PTC) remains unclear. Here, we found that ERα and ERβ were co-expressed in human PTC tissues and cells. ERα promoted and ERβ inhibited the proliferation, invasion and migration of PTC cells. PES1 modulated the balance between ERα and ERβ by elevating the ERα protein level and simultaneously reducing the ERβ protein level, then upregulating the ERα/ERβ protein ratio and promoting the proliferation, invasion and migration of PTC cells. In PTC tissues, PES1 protein level was positively correlated with the ERα protein level and negatively correlated with the ERβ protein level. The PES1 and ERα protein levels were gradually increased and the ERβ protein level was decreased by degree in the occurrence and development of PTC. Increased PES1 and ERα protein levels and decreased ERβ protein level were correlated with the aggressive behaviors of PTC patients such as large tumor size, extrathyroidal extension (ETE), lymph node metastasis (LNM), high BRAFV600E expression and high TNM stage. It is suggested that PES1 promotes the occurrence and development of PTC by elevating the ERα protein level and reducing the ERβ protein level, and then upregulating the ERα/ERβ protein ratio.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

