Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis
- PMID: 30705514
- PMCID: PMC6348411
- DOI: 10.3164/jcbn.18-47
Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis
Abstract
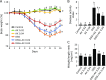
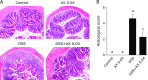
Astaxanthin is a xanthophyll carotenoid, which possesses strong scavenging effect on reactive oxygen species. In this study, we examined the effect of astaxanthin on dextran sulfate sodium (DSS)-induced colitis in mice. Experimental colitis was induced by the oral administration of 4% w/v DSS in tap water in C57BL/6J mice. Astaxanthin was mixed with a normal rodent diet (0.02 or 0.04%). Astaxanthin significantly ameliorated DSS-induced body weight loss and reduced the disease activity index. The ameliorating effects was observed in a dose-dependent manner. Immunochemical analyses showed that astaxanthin markedly suppressed DSS-induced histological inflammatory changes (inflammatory cell infiltration, edematous changes and goblet cell depletion). Plasma levels of malondialdehyde and 8-hydroxy-2-deoxyguanosine were significantly reduced by the administration of 0.04% astaxanthin. Astaxanthin significantly suppressed the mucosal mRNA expression of IL-1β, IL-6, TNF-α, IL-36α and IL-36γ. Astaxanthin blocked the DSS-induced translocation of NF-κB p65 and AP-1 (c-Jun) into the nucleus of mucosal epithelial cells, and also suppressed DSS-induced mucosal activation of MAPKs (ERK1/2, p38 and JNK). In conclusion, astaxanthin prevented the development of DSS-induced colitis via the direct suppression of NF-κB, AP-1 and MAPK activation. These findings suggest that astaxanthin is a novel candidate as a therapeutic option for the treatment of inflammatory bowel disease.
Keywords: carotenoid; inflammatory bowel disease; reactive oxygen species.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures





Similar articles
-
Ameliorating effects of bortezomib, a proteasome inhibitor, on development of dextran sulfate sodium-induced murine colitis.J Clin Biochem Nutr. 2018 Nov;63(3):217-223. doi: 10.3164/jcbn.18-42. Epub 2018 Jun 8. J Clin Biochem Nutr. 2018. PMID: 30487672 Free PMC article.
-
Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice.J Ethnopharmacol. 2017 Feb 23;198:389-398. doi: 10.1016/j.jep.2017.01.042. Epub 2017 Jan 22. J Ethnopharmacol. 2017. PMID: 28119098
-
Andrographolide Derivative AL-1 Ameliorates Dextran Sodium Sulfate-Induced Murine Colitis by Inhibiting NF-κB and MAPK Signaling Pathways.Oxid Med Cell Longev. 2019 Oct 7;2019:6138723. doi: 10.1155/2019/6138723. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31687082 Free PMC article.
-
Structures of Astaxanthin and Their Consequences for Therapeutic Application.Int J Food Sci. 2020 Jul 20;2020:2156582. doi: 10.1155/2020/2156582. eCollection 2020. Int J Food Sci. 2020. PMID: 32775406 Free PMC article. Review.
-
Astaxanthin as a Potential Protector of Liver Function: A Review.J Clin Med Res. 2016 Oct;8(10):701-4. doi: 10.14740/jocmr2672w. Epub 2016 Aug 30. J Clin Med Res. 2016. PMID: 27635173 Free PMC article. Review.
Cited by
-
Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy.Mar Drugs. 2019 Sep 23;17(10):546. doi: 10.3390/md17100546. Mar Drugs. 2019. PMID: 31547619 Free PMC article. Review.
-
Recent advances in anti-inflammatory active components and action mechanisms of natural medicines.Inflammopharmacology. 2023 Dec;31(6):2901-2937. doi: 10.1007/s10787-023-01369-9. Epub 2023 Nov 10. Inflammopharmacology. 2023. PMID: 37947913 Review.
-
Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants.Nutrients. 2021 Dec 27;14(1):107. doi: 10.3390/nu14010107. Nutrients. 2021. PMID: 35010981 Free PMC article. Review.
-
Ibrutinib attenuated DSS-induced ulcerative colitis, oxidative stress, and the inflammatory cascade by modulating the PI3K/Akt and JNK/NF-κB pathways.Arch Med Sci. 2022 Apr 20;18(3):805-815. doi: 10.5114/aoms/146792. eCollection 2022. Arch Med Sci. 2022. PMID: 35591835 Free PMC article. No abstract available.
-
Apigenin-Mn(II) loaded hyaluronic acid nanoparticles for ulcerative colitis therapy in mice.Front Chem. 2022 Jul 22;10:969962. doi: 10.3389/fchem.2022.969962. eCollection 2022. Front Chem. 2022. PMID: 35936086 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

