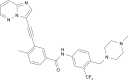
Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies
- PMID: 30705592
- PMCID: PMC6343508
- DOI: 10.2147/OTT.S189391
Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies
Abstract
Human malignancies are often the result of overexpressed and constitutively active receptor and non-receptor tyrosine kinases, which ultimately lead to the mediation of key tumor-driven pathways. Several tyrosine kinases (ie, EGFR, FGFR, PDGFR, VEGFR), are aberrantly activated in most common tumors, including leukemia, glioblastoma, gastrointestinal stromal tumors, non-small-cell lung cancer, and head and neck cancers. Iclusig™ (ponatinib, previously known as AP24534) is an orally active multi-tyrosine kinase inhibitor and is currently approved by the US Food and Drug Administration for patients with chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia, specifically targeting the BCR-ABL gene mutation, T315I. Due to ponatinib's unique multi-targeted characteristics, further studies have demonstrated its ability to target other important tyrosine kinases (FGFR, PDGFR, SRC, RET, KIT, and FLT1) in other human malignancies. This review focuses on the available data of ponatinib and its molecular targets for treatment in various cancers, with a discussion on the broader potential of this agent in other cancer indications.
Keywords: cancer treatment; multi-kinase inhibitor; ponatinib; repurposing.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. - PubMed
-
- Porta R, Borea R, Coelho A, et al. FGFR a promising druggable target in cancer: molecular biology and new drugs. Crit Rev Oncol Hematol. 2017;113:256–267. - PubMed
-
- Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist. 2005;10(7):461–466. - PubMed
-
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21(12):2237–2246. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous