pH as a potential therapeutic target to improve temozolomide antitumor efficacy : A mechanistic modeling study
- PMID: 30705757
- PMCID: PMC6349072
- DOI: 10.1002/prp2.454
pH as a potential therapeutic target to improve temozolomide antitumor efficacy : A mechanistic modeling study
Abstract
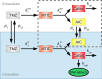
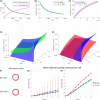
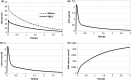
Despite intensive treatments including temozolomide (TMZ) administration, glioblastoma patient prognosis remains dismal and innovative therapeutic strategies are urgently needed. A systems pharmacology approach was undertaken to investigate TMZ pharmacokinetics-pharmacodynamics (PK-PD) incorporating the effect of local pH, tumor spatial configuration and micro-environment. A hybrid mathematical framework was designed coupling ordinary differential equations describing the intracellular reactions, with a spatial cellular automaton to individualize the cells. A differential drug impact on tumor and healthy cells at constant extracellular pH was computationally demonstrated as TMZ-induced DNA damage was larger in tumor cells as compared to normal cells due to less acidic intracellular pH in cancer cells. Optimality of TMZ efficacy defined as maximum difference between damage in tumor and healthy cells was reached for extracellular pH between 6.8 and 7.5. Next, TMZ PK-PD in a solid tumor was demonstrated to highly depend on its spatial configuration as spread cancer cells or fragmented tumors presented higher TMZ-induced damage as compared to compact tumor spheroid. Simulations highlighted that smaller tumors were less acidic than bigger ones allowing for faster TMZ activation and their closer distance to blood capillaries allowed for better drug penetration. For model parameters corresponding to U87 glioma cells, inter-cell variability in TMZ uptake play no role regarding the mean drug-induced damage in the whole cell population whereas this quantity was increased by inter-cell variability in TMZ efflux which was thus a disadvantage in terms of drug resistance. Overall, this study revealed pH as a new potential target to significantly improve TMZ antitumor efficacy.
Keywords: glioblastoma; mathematical modeling; pH; pharmacokinetics‐pharmacodynamics; temozolomide.
Figures






References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987‐996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

