Muscadine grape skin extract inhibits prostate cancer cells by inducing cell-cycle arrest, and decreasing migration through heat shock protein 40
- PMID: 30705983
- PMCID: PMC6348279
- DOI: 10.1016/j.heliyon.2019.e01128
Muscadine grape skin extract inhibits prostate cancer cells by inducing cell-cycle arrest, and decreasing migration through heat shock protein 40
Abstract
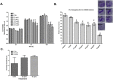
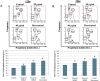
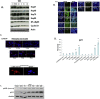
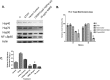
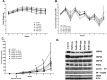
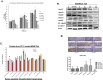
Previously we demonstrated that muscadine grape skin extract (MSKE), a natural product, significantly inhibited androgen-responsive prostate cancer cell growth by inducing apoptosis through the targeting of survival pathways. However, the therapeutic effect of MSKE on more aggressive androgen-independent prostate cancer remains unknown. This study examined the effects of MSKE treatment in metastatic prostate cancer using complementary PC-3 cells and xenograft model. MSKE significantly inhibited PC-3 human prostate cancer cell tumor growth in vitro and in vivo. The growth-inhibitory effect of MSKE appeared to be through the induction of cell-cycle arrest. This induction was accompanied by a reduction in the protein expression of Hsp40 and cell-cycle regulation proteins, cyclin D1 and NF-kBp65. In addition, MSKE induced p21 expression independent of wild-type p53 induced protein expression. Moreover, we demonstrate that MSKE significantly inhibited cell migration in PC-3 prostate cancer cells. Overall, these results demonstrate that MSKE inhibits prostate tumor growth and migration, and induces cell-cycle arrest by targeting Hsp40 and proteins involved in cell-cycle regulation and proliferation. This suggests that MSKE may also be explored either as a neo-adjuvant or therapeutic for castration resistant prostate cancer.
Keywords: Cancer research; Cell biology; Molecular biology.
Figures




Similar articles
-
Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms.Cancer Res. 2007 Sep 1;67(17):8396-405. doi: 10.1158/0008-5472.CAN-06-4069. Cancer Res. 2007. PMID: 17804756
-
Muscadine Grape Skin Extract Induces an Unfolded Protein Response-Mediated Autophagy in Prostate Cancer Cells: A TMT-Based Quantitative Proteomic Analysis.PLoS One. 2016 Oct 18;11(10):e0164115. doi: 10.1371/journal.pone.0164115. eCollection 2016. PLoS One. 2016. PMID: 27755556 Free PMC article.
-
Muscadine grape skin extract reverts snail-mediated epithelial mesenchymal transition via superoxide species in human prostate cancer cells.BMC Complement Altern Med. 2014 Mar 12;14:97. doi: 10.1186/1472-6882-14-97. BMC Complement Altern Med. 2014. PMID: 24617993 Free PMC article.
-
Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells.Carcinogenesis. 2015 Sep;36(9):1019-27. doi: 10.1093/carcin/bgv084. Epub 2015 Jun 10. Carcinogenesis. 2015. PMID: 26069256 Free PMC article.
-
Natural compound Alternol as a novel therapeutic for prostate cancer treatment.Am J Clin Exp Urol. 2020 Jun 25;8(3):76-80. eCollection 2020. Am J Clin Exp Urol. 2020. PMID: 32699806 Free PMC article. Review.
Cited by
-
Grape Powder Supplementation Attenuates Prostate Neoplasia Associated with Pten Haploinsufficiency in Mice Fed High-Fat Diet.Mol Nutr Food Res. 2020 Aug;64(16):e2000326. doi: 10.1002/mnfr.202000326. Epub 2020 Jul 12. Mol Nutr Food Res. 2020. PMID: 32618118 Free PMC article.
-
Natural Health Products (NHP's) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends.Int J Mol Sci. 2020 Nov 11;21(22):8480. doi: 10.3390/ijms21228480. Int J Mol Sci. 2020. PMID: 33187200 Free PMC article. Review.
-
A Polyphenol-Rich Extract From Muscadine Grapes Inhibits Triple-Negative Breast Tumor Growth.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420917444. doi: 10.1177/1534735420917444. Integr Cancer Ther. 2020. PMID: 32578460 Free PMC article.
-
Long-read, chromosome-scale assembly of Vitis rotundifolia cv. Carlos and its unique resistance to Xylella fastidiosa subsp. fastidiosa.BMC Genomics. 2023 Jul 20;24(1):409. doi: 10.1186/s12864-023-09514-y. BMC Genomics. 2023. PMID: 37474911 Free PMC article.
-
Heat Shock Proteins in Benign Prostatic Hyperplasia and Prostate Cancer.Int J Mol Sci. 2022 Jan 14;23(2):897. doi: 10.3390/ijms23020897. Int J Mol Sci. 2022. PMID: 35055079 Free PMC article. Review.
References
-
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. https://www.sciencedirect.com/science/article/pii/S0024320599004105?via%... - PubMed
-
- Clinton S.K. Diet, nutrition, and prostate cancer. Annu. Rev. Nutr. 1998;18:413–440. https://www.annualreviews.org/doi/10.1146/annurev.nutr.18.1.413 - DOI - PubMed
-
- Syed D.N., Khan N., Afaq F., Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol. Biomark. Prev. 2007;16:2193–2203. http://cebp.aacrjournals.org/content/16/11/2193 - PubMed
-
- Thomasset S.C., Berry D.P., Garcea G., Marczylo T., Steward W.P., Gescher A.J. Dietary polyphenolic phytochemical – promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2006;120:451–458. https://onlinelibrary.wiley.com/doi/abs/10.1002/ijc.22419 - DOI - PubMed
-
- Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high grade prostate intraepithelial neoplasia: a preliminary report from a one year proof-of-principle study. Can. Res. 2006;66:1234–1240. http://cancerres.aacrjournals.org/content/66/2/1234 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

