Dynamic of the somatosensory system in postherpetic neuralgia
- PMID: 30706032
- PMCID: PMC6344136
- DOI: 10.1097/PR9.0000000000000668
Dynamic of the somatosensory system in postherpetic neuralgia
Abstract
Introduction: In postherpetic neuralgia (PHN) different types of patients can be distinguished regarding their predominant peripheral nociceptor function.
Objective: The aim was to examine somatosensory profiles in the course of disease with special regard to the different subtypes existing in PHN.
Methods: Twenty patients with PHN (7 men and 13 women, age 67 ± 9.6 years) were examined at baseline (disease duration 18.1 ± 26 months) and follow-up (31.6 ± 23.8 months later) with quantitative sensory testing (protocol of the German Research Network on Neuropathic Pain).
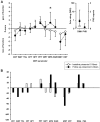
Results: Fourteen (70%) PHN patients presented with impaired (iPHN) and 6 (30%) with preserved (pPHN) C-fiber function. Groups did not differ regarding age, disease duration, or pain intensity at baseline. Both groups did not differ regarding change in pain intensity (-0.5 ± 2.3 vs -1.7 ± 2.6 numerical rating scale, P = n.s.) at follow-up. Impaired PHN improved in thermal and mechanical detection thresholds as well as allodynia independent from change in pain intensity. By contrast, pPHN showed an increase in mechanical pain sensitivity (1.4 ± 2.5 vs -0.4 ± 2.2, P < 0.05) and a trend towards a stronger loss of detection (66% vs 33%, P = n.s.) on follow-up.
Conclusion: Results demonstrate that patients with preserved C-fiber function are more predisposed to develop signs of central sensitization as demonstrated by an increased mechanical pain sensitivity. Impaired C-fiber function is able to improve even in chronic cases, but a functional loss is unlikely to play a role here. The knowledge of development of somatosensory profiles in the course of the disease offers possibilities to optimize a mechanism-based treatment.
Keywords: Chronic pain; Mechanism-based treatment; Postherpetic neuralgia; QST; Somatosensory profiles.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Baron R. Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Clin Pract Neurol 2006;2:95–106. - PubMed
-
- Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol 2013;74:630–6. - PubMed
-
- Boogaard S, Heymans MW, de Vet HC, Peters ML, Loer SA, Zuurmond WW, Perez RS. Predictors of persistent neuropathic pain—a systematic review. Pain Physician 2015;18:433–57. - PubMed
-
- Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain 2017;18:359.e1–359.e38. - PubMed
-
- Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, Jensen TS, Sindrup SH. Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: a randomised, double-blind, and placebo-controlled, phenotype panel study. PAIN 2015;156:2234–44. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
