Prenatal cell-free DNA screening for fetal aneuploidy in pregnant women at average or high risk: Results from a large US clinical laboratory
- PMID: 30706702
- PMCID: PMC6418367
- DOI: 10.1002/mgg3.545
Prenatal cell-free DNA screening for fetal aneuploidy in pregnant women at average or high risk: Results from a large US clinical laboratory
Abstract
Background: We evaluated the performance of a cell-free DNA (cfDNA) prenatal screening assay for trisomies 21, 18, and 13, and sex chromosome aneuploidies (SCAs) among a population of pregnant women that included both those at average and high risk.
Methods: Specimen collection, cfDNA extraction, massively parallel sequencing, and bioinformatics analysis were conducted per laboratory protocol. Assay results, concordance with pregnancy outcomes, and performance characteristics were evaluated.
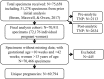
Results: A total 75,658 specimens from 72,176 individual pregnant women were received. Technical reasons accounted for 288 (0.4% of all received samples) tests not performed. In the final analysis cohort (N = 69,794), 13% of pregnancies were considered at average risk and 87% at high risk. Mean gestational age at specimen collection was 15.1 weeks. Of the 69,794 unique pregnancies, 1,359 (1.9%) had positive test results. Among the results with confirmed outcomes, PPV for trisomies 21, 18, and 13 was 98.1%, 88.2%, and 59.3%, respectively; the PPV was 69.0% for SCAs and 75.0% for microdeletions. Overall, PPV was 87.2%, sensitivity was 97.9%, and specificity was 99.9%.
Conclusion: This cfDNA prenatal screening assay provides highly accurate discrimination between affected and unaffected pregnancies among a population of pregnant women at average and high risk for fetal genetic abnormalities.
Keywords: cfDNA prenatal screening assay; fetal aneuploidy; genetic counseling; microdeletion; microduplication; positive predictive value; sex chromosome aneuploidy; trisomy 13; trisomy 18; trisomy 21.
© 2019 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
All authors are employees of Quest Diagnostics.
Figures
Similar articles
-
DNA sequencing versus standard prenatal aneuploidy screening.N Engl J Med. 2014 Feb 27;370(9):799-808. doi: 10.1056/NEJMoa1311037. N Engl J Med. 2014. PMID: 24571752
-
Performance of prenatal cfDNA screening for sex chromosomes.Genet Med. 2023 Aug;25(8):100879. doi: 10.1016/j.gim.2023.100879. Epub 2023 May 5. Genet Med. 2023. PMID: 37154148
-
Prenatal cell-free DNA screening for chromosomal aneuploidies after euploid embryo transfer shows high concordance with preimplantation genetic testing for aneuploidy results and low positive predictive values.Fertil Steril. 2024 Dec;122(6):1105-1113. doi: 10.1016/j.fertnstert.2024.07.029. Epub 2024 Jul 27. Fertil Steril. 2024. PMID: 39069216
-
Cell-Free DNA Screening for Aneuploidy and Microdeletion Syndromes.Obstet Gynecol Clin North Am. 2018 Mar;45(1):13-26. doi: 10.1016/j.ogc.2017.10.001. Obstet Gynecol Clin North Am. 2018. PMID: 29428281 Review.
-
Cell-free DNA for the detection of fetal aneuploidy.Fertil Steril. 2018 Feb;109(2):195-200. doi: 10.1016/j.fertnstert.2017.12.019. Fertil Steril. 2018. PMID: 29447662 Review.
Cited by
-
Microbial cell-free DNA-sequencing as an addition to conventional diagnostics in neonatal sepsis.Pediatr Res. 2025 Feb;97(2):614-624. doi: 10.1038/s41390-024-03448-1. Epub 2024 Aug 14. Pediatr Res. 2025. PMID: 39143203 Free PMC article.
-
Non-invasive prenatal test findings in 41,819 pregnant women: results from a clinical laboratory in southern China.Arch Gynecol Obstet. 2023 Sep;308(3):787-795. doi: 10.1007/s00404-022-06908-3. Epub 2023 Jan 5. Arch Gynecol Obstet. 2023. PMID: 36602559 Review.
-
Parental questions about sex chromosome aneuploidies regarding sex, gender, and sexual orientation as reported by genetic counselors in a prenatal setting.J Genet Couns. 2025 Feb;34(1):e1897. doi: 10.1002/jgc4.1897. Epub 2024 Apr 12. J Genet Couns. 2025. PMID: 38610065 Free PMC article.
-
Omics Approaches to Study Formation and Function of Human Placental Syncytiotrophoblast.Front Cell Dev Biol. 2021 Jun 15;9:674162. doi: 10.3389/fcell.2021.674162. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34211975 Free PMC article. Review.
-
Fundamental origins of neural tube defects with a basis in genetics and nutrition.Exp Brain Res. 2025 Mar 2;243(4):79. doi: 10.1007/s00221-025-07016-9. Exp Brain Res. 2025. PMID: 40025180 Review.
References
-
- American College of Obstetricians and Gynecologists (2007). ACOG practice bulletin no. 77: Screening for fetal chromosomal abnormalities. Obstetrics & Gynecology, 109, 217–227. - PubMed
-
- Bianchi, D. W. , Parsa, S. , Bhatt, S. , Halks‐Miller, M. , Kurtzman, K. , Sehnert, A. J. , & Swanson, A. (2015). Fetal sex chromosome testing by maternal plasma DNA sequencing: Clinical laboratory experience and biology. Obstetrics & Gynecology, 125, 375–382. 10.1097/AOG.0000000000000637 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical