Substrate-Controlled Direct α-Stereoselective Synthesis of Deoxyglycosides from Glycals Using B(C6F5)3 as Catalyst
- PMID: 30706711
- PMCID: PMC6466476
- DOI: 10.1021/acs.joc.8b02613
Substrate-Controlled Direct α-Stereoselective Synthesis of Deoxyglycosides from Glycals Using B(C6F5)3 as Catalyst
Abstract
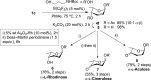
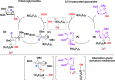
B(C6F5)3 enables the metal-free unprecedented substrate-controlled direct α-stereoselective synthesis of deoxyglycosides from glycals. 2,3-Unsaturated α- O-glycoside products are obtained with deactivated glycals at 75 °C in the presence of the catalyst, while 2-deoxyglycosides are formed using activated glycals that bear no leaving group at C-3 at lower temperatures. The reaction proceeds in good to excellent yields via concomitant borane activation of glycal donor and nucleophile acceptor. The method is exemplified with the synthesis of a series of rare and biologically relevant glycoside analogues.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

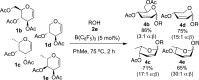

References
-
- Marzabadi C. H.; Franck R. W. The synthesis of 2-deoxyglycosides: 1988–1999. Tetrahedron 2000, 56, 8385–8417. 10.1016/S0040-4020(00)00691-8. - DOI
- Williams R.; Galan M. C. Recent Advances in Organocatalytic Glycosylations. Eur. J. Org. Chem. 2017, 2017, 6247–6264. 10.1002/ejoc.201700785. - DOI
- Medina S.; Galan M. C. Recent developments in the stereoselective synthesis of deoxy glycosides. Carbohydr. Chem. 2015, 41, 59–89. 10.1039/9781782620600-00059. - DOI
-
- Benito-Alifonso D.; Galan M. C.. Bronsted and Lewis Acid Catalyzed Glycosylation. In Selective Glycosylations: Synthetic Methods and Catalysis; Bennett C. E., Ed.; 2017; pp 155–171.
- McKay M. J.; Nguyen H. M. Recent Advances in Transition Metal-Catalyzed Glycosylation. ACS Catal. 2012, 2, 1563–1595. 10.1021/cs3002513. - DOI - PMC - PubMed
- Balmond E. I.; Galan M. C.; McGarrigle E. M. Recent Developments in the Application of Organocatalysis to Glycosylations. Synlett 2013, 24, 2335–2339. 10.1055/s-0033-1338970. - DOI
- Li X. H.; Zhu J. L. Glycosylation via Transition-Metal Catalysis: Challenges and Opportunities. Eur. J. Org. Chem. 2016, 2016, 4724–4767. and references therein10.1002/ejoc.201600484. - DOI
- Pradhan T. K.; Lin C. C.; Mong K. K. T. Preparation of a Protected 3-Deoxy-D-manno-oct-2-ulosonate Glycal Donor for the Synthesis of beta-KDO-Containing Oligosaccharides. Org. Lett. 2014, 16, 1474–1477. 10.1021/ol500275j. - DOI - PubMed
- Wang H.; Tao J. Y.; Cai X. P.; Chen W.; Zhao Y. Q.; Xu Y.; Yao W.; Zeng J.; Wan Q. Stereoselective Synthesis of alpha-Linked 2-Deoxy Glycosides Enabled by Visible-Light-Mediated Reductive Deiodination. Chem. - Eur. J. 2014, 20, 17319–17323. 10.1002/chem.201405516. - DOI - PubMed
- Song W. Z.; Zhao Y.; Lynch J. C.; Kim H.; Tang W. P. Divergent de novo synthesis of all eight stereoisomers of 2,3,6-trideoxyhexopyranosides and their oligomers. Chem. Commun. 2015, 51, 17475–17478. 10.1039/C5CC07787G. - DOI - PubMed
- Thombal R. S.; Jadhav V. H. Facile O-glycosylation of glycals using Glu-Fe3O4-SO3H, a magnetic solid acid catalyst. RSC Adv. 2016, 6, 30846–30851. 10.1039/C6RA03305A. - DOI
- Tanaka H.; Yoshizawa A.; Takahashi T. Direct and stereoselective synthesis of beta-linked 2,6-deoxyoligosaccharides. Angew. Chem., Int. Ed. 2007, 46, 2505–2507. 10.1002/anie.200604031. - DOI - PubMed
- Verma V. P.; Wang C. C. Highly Stereoselective Glycosyl-Chloride-Mediated Synthesis of 2-Deoxyglucosides. Chem. - Eur. J. 2013, 19, 846–851. 10.1002/chem.201203418. - DOI - PubMed
- Zhu D. Y.; Baryal K. N.; Adhikari S.; Zhu J. L. Direct Synthesis of 2-Deoxy-beta-Glycosides via Anomeric O-Alkylation with Secondary Electrophiles. J. Am. Chem. Soc. 2014, 136, 3172–3175. 10.1021/ja4116956. - DOI - PubMed
- Beale T. M.; Moon P. J.; Taylor M. S. Organoboron-Catalyzed Regio- and Stereoselective Formation of beta-2-Deoxyglycosidic Linkages. Org. Lett. 2014, 16, 3604–3607. 10.1021/ol501711v. - DOI - PubMed
- Soliman S. E.; Bennett C. S. Reagent-Controlled Synthesis of the Branched Trisaccharide Fragment of the Antibiotic Saccharomicin B. Org. Lett. 2018, 20, 3413–3416. 10.1021/acs.orglett.8b01355. - DOI - PMC - PubMed
- Lloyd D.; Bennett C. S. An Improved Approach to the Direct Construction of 2-Deoxy-beta-Linked Sugars: Applications to Oligosaccharide Synthesis. Chem. - Eur. J. 2018, 24, 7610–7614. 10.1002/chem.201800736. - DOI - PMC - PubMed
- Bennett C. S.; Galan M. C. Methods for 2-Deoxyglycoside Synthesis. Chem. Rev. 2018, 118, 7931–7985 . and references therein10.1021/acs.chemrev.7b00731. - DOI - PMC - PubMed
- Bradshaw G. A.; Colgan A. C.; Allen N. P.; Pongener I.; Boland M. B.; Ortin Y.; McGarrigle E. M. Stereoselective organocatalyzed glycosylations - thiouracil, thioureas and monothiophthalimide act as Bronsted acid catalysts at low loadings. Chem. Sci. 2019, 10, 508–514. 10.1039/C8SC02788A. - DOI - PMC - PubMed
-
- Zhao G. Y.; Wang T. Stereoselective Synthesis of 2-Deoxyglycosides from Glycals by Visible-Light-Induced Photoacid Catalysis. Angew. Chem., Int. Ed. 2018, 57, 6120–6124. 10.1002/anie.201800909. - DOI - PubMed
- Medina S.; Harper M. J.; Balmond E. I.; Miranda S.; Crisenza G. E. M.; Coe D. M.; McGarrigle E. M.; Galan M. C. Stereoselective Glycosylation of 2-Nitrogalactals Catalyzed by a Bifunctional Organocatalyst. Org. Lett. 2016, 18, 4222–4225. 10.1021/acs.orglett.6b01962. - DOI - PMC - PubMed
- Balmond E. I.; Benito-Alifonso D.; Coe D. M.; Alder R. W.; McGarrigle E. M.; Galan M. C. A 3,4-trans-Fused Cyclic Protecting Group Facilitates alpha-Selective Catalytic Synthesis of 2-Deoxyglycosides. Angew. Chem., Int. Ed. 2014, 53, 8190–8194. 10.1002/anie.201403543. - DOI - PMC - PubMed
- Balmond E. I.; Coe D. M.; Galan M. C.; McGarrigle E. M. Alpha-Selective Organocatalytic Synthesis of 2-Deoxygalactosides. Angew. Chem., Int. Ed. 2012, 51, 9152–9155. 10.1002/anie.201204505. - DOI - PubMed
- Sherry B. D.; Loy R. N.; Toste F. D. Rhenium(V)-catalyzed synthesis of 2-deoxy-alpha-glycosides. J. Am. Chem. Soc. 2004, 126, 4510–4511. 10.1021/ja031895t. - DOI - PubMed
- Das S.; Pekel D.; Neudorfl J. M.; Berkessel A. Organocatalytic Glycosylation by Using Electron- Deficient Pyridinium Salts. Angew. Chem., Int. Ed. 2015, 54, 12479–12483. 10.1002/anie.201503156. - DOI - PubMed
- Liu J. L.; Zhang Y. T.; Liu H. F.; Zhou L.; Chen J. N-Heterocyclic Carbene Catalyzed Stereoselective Glycosylation of 2-Nitrogalactals. Org. Lett. 2017, 19, 5272–5275. 10.1021/acs.orglett.7b02543. - DOI - PubMed
-
- Schmidt R. R.; Angerbauer R. Convenient Preparation of 2,3-Unsaturated N-Galactosyl Derivatives. Carbohydr. Res. 1979, 72, 272–275. 10.1016/S0008-6215(00)83948-8. - DOI
- Fraser-Reid B. Some progeny of 2,3-unsaturated sugars - They little resemble grandfather glucose: Twenty years later. Acc. Chem. Res. 1996, 29, 57–66. 10.1021/ar950104s. - DOI
- Csuk R.; Schaade M.; Krieger C. Synthesis of C-glycosides from glycals or vinylogous lactones and trimethylsilyl ketene acetals. Tetrahedron 1996, 52, 6397–6408. 10.1016/0040-4020(96)00275-X. - DOI
- Borrachero-Moya P.; Cabrera-Escribano F.; Gomez-Guillen M.; Paredes-Leon M. d. R. Synthesis of 4-(4,6-di-0-benzyl-2,3-dideoxy-beta-D-erythro-hex-2-enopyranosyl)pyrazoles from 3,4,6-tri-O-acetyl-D-glucal. Carbohydr. Res. 1998, 308, 181–190. 10.1016/S0008-6215(98)00059-7. - DOI
- Gomez A. M.; Lobo F.; Uriel C.; Lopez J. C. Recent Developments in the Ferrier Rearrangement. Eur. J. Org. Chem. 2013, 2013, 7221–7262. 10.1002/ejoc.201300798. - DOI
- Ferrier R. J. Unsaturated Carbohydrates. Part II. Three Reactions Leading to Unsaturated Glycopyranosides. J. Chem. Soc. 1964, 5443–5449. 10.1039/jr9640005443. - DOI
- Ferrier R. J.; Hoberg J. O. Synthesis and reactions of unsaturated sugars. Adv. Carbohydr. Chem. Biochem. 2003, 58, 55–119. 10.1016/S0065-2318(03)58003-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

