Late-onset Alzheimer Disease
- PMID: 30707185
- PMCID: PMC6548536
- DOI: 10.1212/CON.0000000000000700
Late-onset Alzheimer Disease
Abstract
Purpose of review: Alzheimer disease (AD) is the most common cause of late-onset dementia. This article describes the epidemiology, genetic and environmental risk factors, clinical diagnosis, biomarkers, and treatment of late-onset AD, defined by age of onset of 65 years or older.
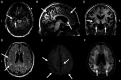
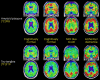
Recent findings: An estimated 5.7 million Americans are living with AD dementia, with the number of affected individuals growing rapidly because of an aging population. Vascular risk factors, sleep disorders, and traumatic brain injury are associated with an increased risk of AD, while increased cognitive and physical activity throughout the lifespan reduce the risk of disease. The primary genetic risk factor for late-onset AD is the apolipoprotein E (APOE) ε4 allele. AD typically presents with early and prominent episodic memory loss, although this clinical syndrome is neither sensitive nor specific for underlying AD neuropathology. Emerging CSF and imaging biomarkers can now detect the key neuropathologic features of the disease (amyloid plaques, neurofibrillary tangles, and neurodegeneration) in living people, allowing for characterization of patients based on biological measures. A comprehensive treatment plan for AD includes use of symptomatic medications, optimal treatment of comorbid conditions and neuropsychiatric symptoms, counseling about safety and future planning, and referrals to community resources.
Summary: AD is very common in older neurologic patients. Neurologists should set the standard for the diagnosis and care of patients with AD and should be familiar with emerging biomarkers that have transformed AD research and are primed to enter the clinical arena.
Figures


References
-
- Alzheimer A. Uber eine eigenaritage, schweren Erkrankung der Hirnrinde. Neurol Zbl 1907;25:1134.
-
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018;14(3):367–429. doi:10.1016/j.jalz.2018.02.001. - PubMed
-
- Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90(3):126–135. doi:10.1212/WNL.0000000000004826. - PMC - PubMed
-
- Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord 2001;15(4):169–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
