Participation of integrin β3 in osteoblast differentiation induced by titanium with nano or microtopography
- PMID: 30707485
- PMCID: PMC7336872
- DOI: 10.1002/jbm.a.36643
Participation of integrin β3 in osteoblast differentiation induced by titanium with nano or microtopography
Abstract
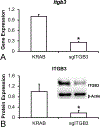
The major role of integrins is to mediate cell adhesion but some of them are involved in the osteoblasts-titanium (Ti) interactions. In this study, we investigated the participation of integrins in osteoblast differentiation induced by Ti with nanotopography (Ti-Nano) and with microtopography (Ti-Micro). By using a PCR array, we observed that, compared with Ti-Micro, Ti-Nano upregulated the expression of five integrins in mesenchymal stem cells, including integrin β3, which increases osteoblast differentiation. Silencing integrin β3, using CRISPR-Cas9, in MC3T3-E1 cells significantly reduced the osteoblast differentiation induced by Ti-Nano in contrast to the effect on T-Micro. Concomitantly, integrin β3 silencing downregulated the expression of integrin αv, the parent chain that combines with other integrins and several components of the Wnt/β-catenin and BMP/Smad signaling pathways, all involved in osteoblast differentiation, only in cells cultured on Ti-Nano. Taken together, our results showed the key role of integrin β3 in the osteogenic potential of Ti-Nano but not of Ti-Micro. Additionally, we propose a novel mechanism to explain the higher osteoblast differentiation induced by Ti-Nano that involves an intricate regulatory network triggered by integrin β3 upregulation, which activates the Wnt and BMP signal transductions. © 2019 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 107A: 1303-1313, 2019.
Keywords: CRISPR; integrin; nanotopography; osteoblast; titanium.
© 2019 Wiley Periodicals, Inc.
Figures








References
-
- de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A 2007;80:554–564. - PubMed
MeSH terms
Substances
Grants and funding
- 2016/21116-5/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2013/05181-3/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/International
- 303464/2016-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico/International
- 2014/08443-1/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
LinkOut - more resources
Full Text Sources
Research Materials

