The renin-angiotensin system: going beyond the classical paradigms
- PMID: 30707614
- PMCID: PMC7191626
- DOI: 10.1152/ajpheart.00723.2018
The renin-angiotensin system: going beyond the classical paradigms
Abstract
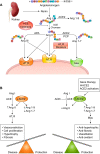
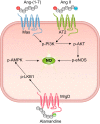
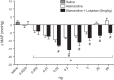
Thirty years ago, a novel axis of the renin-angiotensin system (RAS) was unveiled by the discovery of angiotensin-(1-7) [ANG-(1-7)] generation in vivo. Later, angiotensin-converting enzyme 2 (ACE2) was shown to be the main mediator of this reaction, and Mas was found to be the receptor for the heptapeptide. The functional analysis of this novel axis of the RAS that followed its discovery revealed numerous protective actions in particular for cardiovascular diseases. In parallel, similar protective actions were also described for one of the two receptors of ANG II, the ANG II type 2 receptor (AT2R), in contrast to the other, the ANG II type 1 receptor (AT1R), which mediates deleterious actions of this peptide, e.g., in the setting of cardiovascular disease. Very recently, another branch of the RAS was discovered, based on angiotensin peptides in which the amino-terminal aspartate was replaced by alanine, the alatensins. Ala-ANG-(1-7) or alamandine was shown to interact with Mas-related G protein-coupled receptor D, and the first functional data indicated that this peptide also exerts protective effects in the cardiovascular system. This review summarizes the presentations given at the International Union of Physiological Sciences Congress in Rio de Janeiro, Brazil, in 2017, during the symposium entitled "The Renin-Angiotensin System: Going Beyond the Classical Paradigms," in which the signaling and physiological actions of ANG-(1-7), ACE2, AT2R, and alatensins were reported (with a focus on noncentral nervous system-related tissues) and the therapeutic opportunities based on these findings were discussed.
Keywords: alamandine; angiotensin-(1−7); angiotensin-(1−9); angiotensin-converting enzyme 2; heart failure.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

